Journal of Structural Biology ( IF 3.0 ) Pub Date : 2021-07-01 , DOI: 10.1016/j.jsb.2021.107768 Naruhiko Adachi 1 , Takahide Yamaguchi 2 , Toshio Moriya 3 , Masato Kawasaki 1 , Kotaro Koiwai 3 , Akira Shinoda 3 , Yusuke Yamada 1 , Fumiaki Yumoto 3 , Takamitsu Kohzuma 2 , Toshiya Senda 1
|
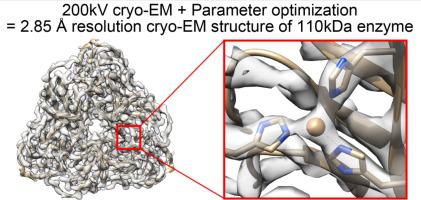
Cu-containing nitrite reductases (NiRs) are 110 kDa enzymes that play central roles in denitrification. Although the NiRs have been well studied, with over 100 Protein Data Bank entries, such issues as crystal packing, photoreduction, and lack of high pH cases have impeded structural analysis of their catalytic mechanisms. Here we show the cryogenic electron microscopy (cryo-EM) structures of Achromobacter cycloclastes NiR (AcNiR) at pH 6.2 and 8.1. The optimization of 3D-reconstruction parameters achieved 2.99 and 2.85 Å resolution. Comprehensive comparisons with cryo-EM and 56 AcNiR crystal structures suggested crystallographic artifacts in residues 185–215 and His255′ due to packing and photoreduction, respectively. We used a newly developed map comparison method to detect structural change around the type 2 Cu site. While the theoretical estimation of coordinate errors of cryo-EM structures remains difficult, combined analysis using X-ray and cryo-EM structures will allow deeper insight into the local structural changes of proteins.
中文翻译:

200 kV 低温电子显微镜测定 110 kDa 亚硝酸还原酶的 2.85 和 2.99 Å 分辨率结构
含铜亚硝酸还原酶 (NiRs) 是 110 kDa 的酶,在反硝化中起核心作用。尽管已经对 NiR 进行了很好的研究,有超过 100 个蛋白质数据库条目,但晶体堆积、光还原和缺乏高 pH 情况等问题阻碍了对其催化机制的结构分析。在这里,我们展示了在 pH 6.2 和 8.1下无色杆菌 cycloclastes NiR ( Ac NiR)的低温电子显微镜 (cryo-EM) 结构。3D 重建参数的优化达到了 2.99 和 2.85 Å 的分辨率。与 cryo-EM 和 56 Ac的综合比较NiR 晶体结构表明,由于堆积和光还原,残基 185-215 和 His255' 中存在晶体学伪影。我们使用新开发的地图比较方法来检测 2 型铜位点周围的结构变化。虽然冷冻电镜结构坐标误差的理论估计仍然很困难,但使用 X 射线和冷冻电镜结构的组合分析将有助于更深入地了解蛋白质的局部结构变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号