Letters in Organic Chemistry ( IF 0.7 ) Pub Date : 2021-07-31 , DOI: 10.2174/1570178617999210104222055 Nasrin Masnabadi 1
|
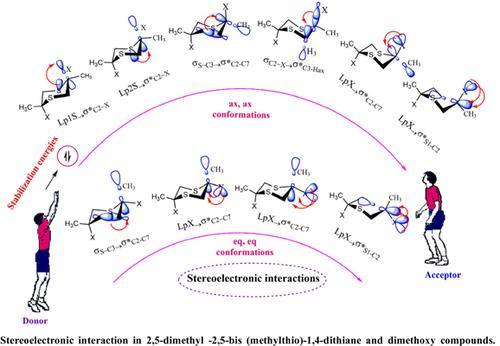
Conformational behaviors of 2, 5-dimethoxy-2,5-dimethyl-1,4-dithiane (compound 1) and 2,5-dimethyl-2,5-bis (methylthio)-1,4-dithiane (compound 2) were investigated by computational methods including B3LYP/6-311+G** and M06-2X/6-311+G** levels of theory and NBO analysis. The stereoelectronic effect of axial, axial (ax, ax) and equatorial, equatorial (eq, eq) conformations was studied using NBO analysis. Using NBO analysis, the values of the stereoelectronic effects were calculated through the energy of stability associated with the electron transfers of compounds 1 and 2. The results showed that the eq, eq conformations of the studied compounds were more stable than their corresponding ax, ax conformations, and LP2X→σS1-C2 and LP2S→σ*C2-X electron transfers play important roles in the conformational behavior of the studied compounds. The main purpose of the present work was to study the effects of stereoelectronic interactions and steric on the conformational superiority of the di-methoxy (compound 1) and di-thiomethyl compounds (compound 2). Thus, the values of resonance stability energy, non-diagonal elements, and orbital populations were investigated. Also, active electrophilic and nucleophilic centers were identified using fronting orbitals analysis obtained by DFT methods. The electrostatic potential maps of the title compounds were investigated at the B3LYP/6-311+G* level of theory. All of the NMR parameters and geometrical properties of both compounds were determined in this study.
中文翻译:

通过 DFT 方法对 2,5-二甲基-2,5-双(甲硫基)-1,4-二噻烷和二甲氧基化合物进行构象稳定性、FMO、NMR、MEP 和 NBO 分析
2, 5-二甲氧基-2,5-二甲基-1,4-二噻烷(化合物1)和2,5-二甲基-2,5-双(甲硫基)-1,4-二噻烷(化合物2)的构象行为为通过包括 B3LYP/6-311+G** 和 M06-2X/6-311+G** 水平理论和 NBO 分析的计算方法进行研究。使用 NBO 分析研究了轴向、轴向 (ax, ax) 和赤道、赤道 (eq, eq) 构象的立体电子效应。使用NBO分析,通过与化合物1和2的电子转移相关的稳定能计算立体电子效应值。结果表明,所研究化合物的eq、eq构象比其对应的ax、ax更稳定构象,LP2X→σS1-C2 和 LP2S→σ*C2-X 电子转移在研究化合物的构象行为中起着重要作用。本工作的主要目的是研究立体电子相互作用和空间对二甲氧基(化合物 1)和二硫甲基化合物(化合物 2)构象优势的影响。因此,研究了共振稳定能、非对角元素和轨道布居的值。此外,使用通过 DFT 方法获得的前向轨道分析鉴定了活性亲电和亲核中心。在 B3LYP/6-311+G* 理论水平上研究了标题化合物的静电势图。在本研究中确定了两种化合物的所有 NMR 参数和几何特性。使用通过 DFT 方法获得的前向轨道分析鉴定了活性亲电和亲核中心。在 B3LYP/6-311+G* 理论水平上研究了标题化合物的静电势图。在本研究中确定了两种化合物的所有 NMR 参数和几何特性。使用通过 DFT 方法获得的前向轨道分析鉴定了活性亲电和亲核中心。在 B3LYP/6-311+G* 理论水平上研究了标题化合物的静电势图。在本研究中确定了两种化合物的所有 NMR 参数和几何特性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号