当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atypically Modified Carbapenem Antibiotics Display Improved Antimycobacterial Activity in the Absence of β-Lactamase Inhibitors
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2021-06-30 , DOI: 10.1021/acsinfecdis.1c00185 Rashmi Gupta 1 , Noora M S A Al-Kharji 2 , Maha A Alqurafi 2 , Thu Q Nguyen 2 , Weirui Chai 2 , Pojun Quan 2 , Riya Malhotra 2 , Breven S Simcox 1 , Phil Mortimer 3 , Leighanne A Brammer Basta 4 , Kyle H Rohde 1 , John D Buynak 2
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2021-06-30 , DOI: 10.1021/acsinfecdis.1c00185 Rashmi Gupta 1 , Noora M S A Al-Kharji 2 , Maha A Alqurafi 2 , Thu Q Nguyen 2 , Weirui Chai 2 , Pojun Quan 2 , Riya Malhotra 2 , Breven S Simcox 1 , Phil Mortimer 3 , Leighanne A Brammer Basta 4 , Kyle H Rohde 1 , John D Buynak 2
Affiliation
|
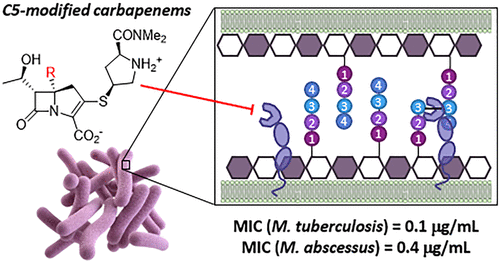
Commercial carbapenem antibiotics are being used to treat multidrug resistant (MDR) and extensively drug resistant (XDR) tuberculosis. Like other β-lactams, carbapenems are irreversible inhibitors of serine d,d-transpeptidases involved in peptidoglycan biosynthesis. In addition to d,d-transpeptidases, mycobacteria also utilize nonhomologous cysteine l,d-transpeptidases (Ldts) to cross-link the stem peptides of peptidoglycan, and carbapenems form long-lived acyl-enzymes with Ldts. Commercial carbapenems are C2 modifications of a common scaffold. This study describes the synthesis of a series of atypical, C5α modifications of the carbapenem scaffold, microbiological evaluation against Mycobacterium tuberculosis (Mtb) and the nontuberculous mycobacterial species, Mycobacterium abscessus (Mab), as well as acylation of an important mycobacterial target Ldt, LdtMt2. In vitro evaluation of these C5α-modified carbapenems revealed compounds with standalone (i.e., in the absence of a β-lactamase inhibitor) minimum inhibitory concentrations (MICs) superior to meropenem-clavulanate for Mtb, and meropenem-avibactam for Mab. Time-kill kinetics assays showed better killing (2–4 log decrease) of Mtb and Mab with lower concentrations of compound 10a as compared to meropenem. Although susceptibility of clinical isolates to meropenem varied by nearly 100-fold, 10a maintained excellent activity against all Mtb and Mab strains. High resolution mass spectrometry revealed that 10a acylates LdtMt2 at a rate comparable to meropenem, but subsequently undergoes an unprecedented carbapenem fragmentation, leading to an acyl-enzyme with mass of Δm = +86 Da. Rationale for the divergence of the nonhydrolytic fragmentation of the LdtMt2 acyl-enzymes is proposed. The observed activity illustrates the potential of novel atypical carbapenems as prospective candidates for treatment of Mtb and Mab infections.
中文翻译:

非典型修饰的碳青霉烯类抗生素在缺乏 β-内酰胺酶抑制剂的情况下显示出更好的抗分枝杆菌活性
商业碳青霉烯类抗生素正被用于治疗耐多药 (MDR) 和广泛耐药 (XDR) 结核病。与其他 β-内酰胺类一样,碳青霉烯类是参与肽聚糖生物合成的丝氨酸d,d-转肽酶的不可逆抑制剂。除了d,d -转肽酶,分枝杆菌还利用非同源半胱氨酸l,d -转肽酶 (Ldts) 来交联肽聚糖的干肽,碳青霉烯类与 Ldts 形成长寿命的酰基酶。商业碳青霉烯是常见支架的 C2 修饰。本研究描述了碳青霉烯支架的一系列非典型 C5α 修饰的合成,以及针对结核分枝杆菌( Mtb) 和非结核分枝杆菌物种,脓肿分枝杆菌( Mab ),以及重要的分枝杆菌目标 Ldt Ldt Mt2 的酰化。对这些 C5α 修饰的碳青霉烯类化合物的体外评估揭示了化合物的独立(即,在没有 β-内酰胺酶抑制剂的情况下)最低抑制浓度 (MIC) 优于美罗培南-克拉维酸对Mtb和美罗培南-阿维巴坦对Mab 的影响。时间杀灭动力学分析显示,化合物10a浓度较低时,Mtb和Mab 的杀灭效果更好(2-4 log 降低)与美罗培南相比。尽管临床分离株对美罗培南的敏感性相差近 100 倍,但10a对所有Mtb和Mab菌株均保持出色的活性。高分辨率质谱显示,10a以与美罗培南相当的速率酰化 Ldt Mt2,但随后经历了前所未有的碳青霉烯碎裂,产生了质量为 Δ m = +86 Da的酰基酶。提出了 Ldt Mt2酰基酶的非水解断裂发散的基本原理。观察到的活性说明了新型非典型碳青霉烯类作为治疗Mtb和单克隆抗体感染。
更新日期:2021-08-13
中文翻译:

非典型修饰的碳青霉烯类抗生素在缺乏 β-内酰胺酶抑制剂的情况下显示出更好的抗分枝杆菌活性
商业碳青霉烯类抗生素正被用于治疗耐多药 (MDR) 和广泛耐药 (XDR) 结核病。与其他 β-内酰胺类一样,碳青霉烯类是参与肽聚糖生物合成的丝氨酸d,d-转肽酶的不可逆抑制剂。除了d,d -转肽酶,分枝杆菌还利用非同源半胱氨酸l,d -转肽酶 (Ldts) 来交联肽聚糖的干肽,碳青霉烯类与 Ldts 形成长寿命的酰基酶。商业碳青霉烯是常见支架的 C2 修饰。本研究描述了碳青霉烯支架的一系列非典型 C5α 修饰的合成,以及针对结核分枝杆菌( Mtb) 和非结核分枝杆菌物种,脓肿分枝杆菌( Mab ),以及重要的分枝杆菌目标 Ldt Ldt Mt2 的酰化。对这些 C5α 修饰的碳青霉烯类化合物的体外评估揭示了化合物的独立(即,在没有 β-内酰胺酶抑制剂的情况下)最低抑制浓度 (MIC) 优于美罗培南-克拉维酸对Mtb和美罗培南-阿维巴坦对Mab 的影响。时间杀灭动力学分析显示,化合物10a浓度较低时,Mtb和Mab 的杀灭效果更好(2-4 log 降低)与美罗培南相比。尽管临床分离株对美罗培南的敏感性相差近 100 倍,但10a对所有Mtb和Mab菌株均保持出色的活性。高分辨率质谱显示,10a以与美罗培南相当的速率酰化 Ldt Mt2,但随后经历了前所未有的碳青霉烯碎裂,产生了质量为 Δ m = +86 Da的酰基酶。提出了 Ldt Mt2酰基酶的非水解断裂发散的基本原理。观察到的活性说明了新型非典型碳青霉烯类作为治疗Mtb和单克隆抗体感染。











































 京公网安备 11010802027423号
京公网安备 11010802027423号