当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Data Analysis for Electrolyte Systems: A Method Illustrated on Alkali Halides in Water
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-06-30 , DOI: 10.1021/acs.jced.1c00105 Santiago Vaque Aura 1 , Juan-Sebastian Roa Pinto 1 , Nicolas Ferrando 1 , Jean-Charles de Hemptinne 1 , Antoon ten Kate 2 , Susanna Kuitunen 3 , Nikolaos Diamantonis 4 , Thomas Gerlach 5 , Manfred Heilig 6 , Gaetan Becker 7 , Mathias Brehelin 8
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-06-30 , DOI: 10.1021/acs.jced.1c00105 Santiago Vaque Aura 1 , Juan-Sebastian Roa Pinto 1 , Nicolas Ferrando 1 , Jean-Charles de Hemptinne 1 , Antoon ten Kate 2 , Susanna Kuitunen 3 , Nikolaos Diamantonis 4 , Thomas Gerlach 5 , Manfred Heilig 6 , Gaetan Becker 7 , Mathias Brehelin 8
Affiliation
|
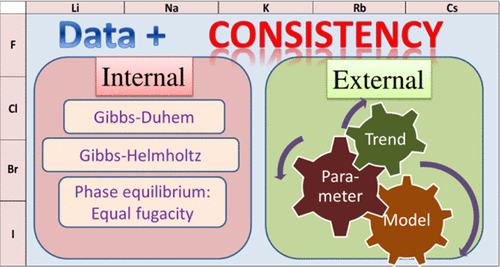
In the recent years, the need to improve the thermodynamic models, particularly making them more precise and predictable for complex systems, has increased [Hendriks, E. Industrial Requirements for Thermodynamics and Transport Properties. Ind. Eng. Chem. Res. 2010, 49, 11131 11141 andKontogeorgis, G. Industrial Requirements for Thermodynamic and Transport Properties. Ind. Eng. Chem. Res. 2021, 60, 4987 5013]. The complexity of electrolyte systems is due to the strong interactions between ions, the hydration forces that take place during salt dissociation, and the physical forces at high concentrations of solute. Consequently, the existing thermodynamic electrolyte models show some limitations and are far from being completely optimized for industrial simulations. Thermodynamics based on electrolyte mixtures and mixed solvents requires further development and research [Poling, B. E. The Properties of Gases and Liquids, 5th ed.; McGraw-Hill Education, 2001].The EleTher Joint Industrial Project (JIP) aims at promoting research in this field. A practical workflow is under development where the first stage consists in analyzing the data. The present work is part of this larger scope and presents a strategy to analyze the internal and external consistency of experimental data for electrolyte mixtures. The focus is on alkali halide salts in water. Internal consistency allows testing the quality of data by confronting them to each other and using the thermodynamic relationships between these data. For that purpose, the data are confronted with a model that was initially optimized and the deviations are analyzed. For this purpose, the electrolyte non-random two-liquid (eNRTL) thermodynamic model was selected. Some deviations are attributed to the model inadequacy (high temperature or high molalities). Others are to be attributed to data inconsistencies. On the other hand, the objective of external consistency is to evaluate the consistency between different systems of the same family. The Bromley model is used for this purpose, as it contains a single adjustable parameter. Investigating the trend of this parameter with type of compound or with temperature may provide valuable information regarding the underlying physics, in addition to identifying outliers.
中文翻译:

电解质系统的数据分析:一种分析水中碱卤化物的方法
近年来,改进热力学模型,特别是使它们对复杂系统更加精确和可预测的需求有所增加。亨德里克斯,E. 热力学和传输特性的工业要求。工业英。化学 水库 2010、49、 11131 11141 和康托乔治斯,G. 热力学和传输特性的工业要求。工业英。化学 水库 2021 , 60 , 4987 5013]。电解质系统的复杂性是由于离子之间的强相互作用、盐解离过程中发生的水合力以及高浓度溶质下的物理力。因此,现有的热力学电解质模型显示出一些局限性,远未完全针对工业模拟进行优化。基于电解质混合物和混合溶剂的热力学需要进一步的发展和研究。波兰, BE 气体和液体的特性,第 5 版;麦格劳-希尔教育, 2001].EleTher 联合工业项目 (JIP) 旨在促进该领域的研究。一个实用的工作流程正在开发中,第一阶段是分析数据。目前的工作是这一更大范围的一部分,并提出了一种分析电解质混合物实验数据内部和外部一致性的策略。重点是水中的碱金属卤化物盐。内部一致性允许通过相互面对数据并使用这些数据之间的热力学关系来测试数据的质量。为此,将数据与最初优化的模型进行对比,并对偏差进行分析。为此,选择了电解质非随机二液 (eNRTL) 热力学模型。一些偏差归因于模型的不足(高温或高摩尔浓度)。其他则归咎于数据不一致。另一方面,外部一致性的目标是评估同一族不同系统之间的一致性。Bromley 模型用于此目的,因为它包含单个可调参数。除了识别异常值外,研究该参数与化合物类型或温度的趋势可能会提供有关基础物理的宝贵信息。
更新日期:2021-08-12
中文翻译:

电解质系统的数据分析:一种分析水中碱卤化物的方法
近年来,改进热力学模型,特别是使它们对复杂系统更加精确和可预测的需求有所增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号