Reactive & Functional Polymers ( IF 5.1 ) Pub Date : 2021-07-01 , DOI: 10.1016/j.reactfunctpolym.2021.104969 Marcia Viltres-Portales , Markel Denet Luaces Alberto , Lei Ye
|
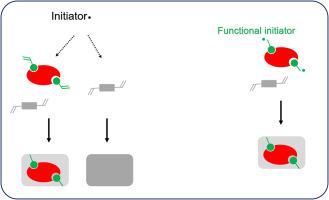
In traditional molecular imprinting reactions, the initial radicals generated by thermo- or photo-decomposition are randomly distributed in the reaction mixture. Because a noticeable portion of the initial radicals is able to cause self-polymerization of the crosslinking monomer, a significant part of the polymer product does not contain successfully imprinted molecular recognition sites. To solve this problem, we designed a molecular imprinting method using functionalized radical initiator to replace the conventional combination of initiator and functional monomer. Since the active radicals in the reaction mixture carry a template-binding moiety, the actual radical polymerization becomes more likely to take place nearby the molecular template. As a result, the efficiency of molecular imprinting can be improved. In this work, we report the use of amidine-functionalized initiator to synthesize molecularly imprinted polymers (MIPs) for selective recognition of methotrexate, a cytostatic drug used for cancer therapy. As glutamic acid represents a substructure of methotrexate, we select to use N-terminal protected glutamic acid as template to synthesize the MIPs. An initiator, 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) is used to provide strong interaction with the molecular template. Two MIPs synthesized using glutamic acid derivatives as templates display specific binding to the fluorescent amino acid derivative Fmoc-Glu. When the molecular binding is tested against methotrexate, the MIP particles also exhibit specific binding for the cytostatic drug. Using cationic functional initiator to target carboxyl epitope of molecular target, this work provides an additional example of molecular imprinting based on functionalized radical initiators.
中文翻译:

使用脒官能化引发剂合成分子印迹聚合物进行羧酸识别
在传统的分子印迹反应中,由热分解或光分解产生的初始自由基随机分布在反应混合物中。由于初始自由基的显着部分能够引起交联单体的自聚合,因此大部分聚合物产品不包含成功印迹的分子识别位点。为了解决这个问题,我们设计了一种使用官能化自由基引发剂来代替引发剂和官能单体的常规组合的分子印迹方法。由于反应混合物中的活性自由基带有模板结合部分,实际自由基聚合更有可能发生在分子模板附近。结果,可以提高分子印记的效率。在这项工作中,我们报告了使用脒功能化引发剂合成分子印迹聚合物 (MIP) 以选择性识别甲氨蝶呤,一种用于癌症治疗的细胞抑制药物。由于谷氨酸代表甲氨蝶呤的亚结构,我们选择使用N-末端受保护的谷氨酸作为模板来合成 MIP。引发剂 2,2'-偶氮双(2-脒基丙烷)二盐酸盐 (AAPH) 用于提供与分子模板的强相互作用。使用谷氨酸衍生物作为模板合成的两个 MIP 显示出与荧光氨基酸衍生物 Fmoc-Glu 的特异性结合。当针对甲氨蝶呤测试分子结合时,MIP 颗粒也表现出对细胞抑制药物的特异性结合。使用阳离子功能引发剂靶向分子靶标的羧基表位,这项工作提供了基于功能化自由基引发剂的分子印记的另一个例子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号