Organic Electronics ( IF 2.7 ) Pub Date : 2021-07-01 , DOI: 10.1016/j.orgel.2021.106273 Ting-Hong Huang , Cheng Luo , Dan Zheng
|
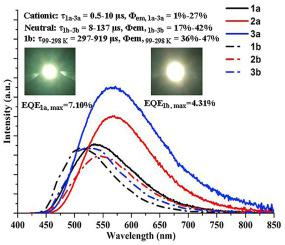
Cationic and neutral mononuclear Cu(I) complexes, [Cu(PPh3)2(PmH)]BF4 (1a), [Cu(DPEphos) (PmH)]BF4 (2a), [Cu(Xantphos) (PmH)]BF4 (3a), [Cu(PPh3)2(Pm)] (1b), [Cu(DPEphos) (Pm)] (2b) and [Cu(Xantphos) (Pm)] (3b) (PPh3 = triphenylphosphine, DPEphos = bis(2-diphenylphosphinophenyl)ether, Xantphos = 9, 9-dimethyl-bis(diphenylphosphino)xanthenes, PmH = 2-(pyridin-2-yl)benzimidazole, Pm=(2-(Pyridin-2-yl)benzimidazolate), have been prepared and characterized by IR, 1H NMR, 13C NMR, 31P NMR, XRD, elemental analysis and X-ray crystal structure analysis. The structural analysis shows that each of Cu(I) complexes includes a tetrahedral [Cu(NN) (PP)]+ moiety, and temperature variation from 99 K to 298 K leads to the change of bonds lengths, angles and weak interactions. Meanwhile, theoretical calculations indicate that the differences between cationic and neutral Cu(I) complexes affect the composition of HOMO and LUMO orbitals, and the effect of temperature on Mülliken atomic charges is limited. Furthermore, neutral Cu(I) complexes 1b–3b show better luminescence in comparison to cationic Cu(I) complexes 1a-3a at room temperature, and temperature variations from 99 K to 298 K result in changing photoluminescence to some extent, which partly agrees with the related calculation results. In these cationic and neutral Cu(I) complexes, the maximum phosphorescent lifetime and quantum yield reach respectively 137 μs and 42% at room temperature. Moreover, cationic and neutral Cu(I) complexes are utilized to fabricate the monochromatic LEDs, showing favorable electroluminescence with the maximum EQE of 7.10%.
中文翻译:

用于发光二极管的发光阳离子/中性 Cu(I) 配合物:合成、结构表征、DFT 研究和特性
阳离子和中性单核 Cu(I) 络合物,[Cu(PPh 3 ) 2 (PmH)]BF 4 (1a),[Cu(DPEphos) (PmH)]BF 4 (2a),[Cu(Xantphos) (PmH) ]BF 4 (3a)、[Cu(PPh 3 ) 2 (Pm)] (1b)、[Cu(DPEphos) (Pm)] (2b) 和 [Cu(Xantphos) (Pm)] (3b) (PPh 3 = 三苯基膦,DPEphos = 双(2-二苯基膦基苯基)醚,Xantphos = 9,9-二甲基-双(二苯基膦基)氧杂蒽,PmH = 2-(吡啶-2-基)苯并咪唑,Pm=(2-(吡啶-2- yl)benzimidazolate), 已制备并通过 IR, 1 H NMR, 13 C NMR, 31P NMR、XRD、元素分析和X射线晶体结构分析。结构分析表明,每个 Cu(I) 配合物都包含一个四面体 [Cu(NN) (PP)] +部分,以及从 99 K 到 298 K 的温度变化导致键长、角度和弱相互作用的变化。同时,理论计算表明,阳离子和中性 Cu(I) 配合物之间的差异影响 HOMO 和 LUMO 轨道的组成,并且温度对 Mülliken 原子电荷的影响是有限的。此外,与阳离子 Cu(I) 配合物 1a-3a 相比,中性 Cu(I) 配合物 1b-3b 在室温下显示出更好的发光,并且从 99 K 到 298 K 的温度变化导致在一定程度上改变光致发光,这部分同意以及相关的计算结果。在这些阳离子和中性 Cu(I) 配合物中,室温下的最大磷光寿命和量子产率分别达到 137 μs 和 42%。而且,











































 京公网安备 11010802027423号
京公网安备 11010802027423号