Chemical & Pharmaceutical Bulletin ( IF 1.5 ) Pub Date : 2021-07-01 , DOI: 10.1248/cpb.c21-00228 Chisato Yoshikawa 1 , Hiroaki Ishida 1 , Nami Ohashi 1 , Hiroyuki Kojima 1 , Toshimasa Itoh 1
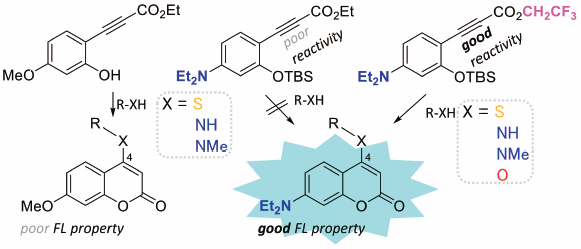
The coumarin skeleton has been a focus of attention for many years, and its fluorescence properties vary depending on the substituents. Fluorescent coumarin derivatives are useful tools for many strategies have been developed for their synthesis. Although 7-diethylaminocoumarin has excellent fluorescence properties, it is unstable. We have developed a facile strategy for the synthesis of 7-diethylaminocoumarin derivatives by increasing the electrophilicity of the ynone moiety to promote nucleophilic addition reactions and cyclization. The reaction tolerates a variety of substitutions at the 4-position.
 Fullsize Image
Fullsize Image
中文翻译:

吸电子基团促进7-二乙基氨基香豆素的构建
香豆素骨架多年来一直是人们关注的焦点,其荧光性质因取代基而异。荧光香豆素衍生物是有用的工具,已开发出许多合成策略。7-二乙基氨基香豆素虽然具有优异的荧光特性,但不稳定。我们通过增加炔酮部分的亲电性来促进亲核加成反应和环化,开发了一种合成 7-二乙基氨基香豆素衍生物的简便策略。该反应耐受 4 位的多种取代。
 全尺寸图像
全尺寸图像











































 京公网安备 11010802027423号
京公网安备 11010802027423号