当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal structure of human brain-type fatty acid-binding protein FABP7 complexed with palmitic acid
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2021-06-29 , DOI: 10.1107/s2059798321005763 Ki Hyun Nam 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2021-06-29 , DOI: 10.1107/s2059798321005763 Ki Hyun Nam 1
Affiliation
|
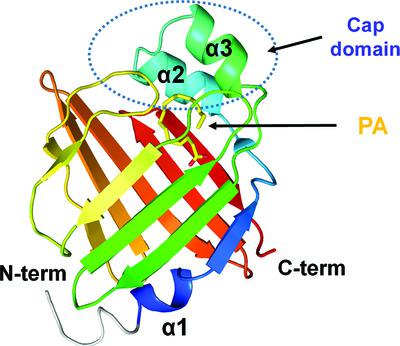
The brain-type fatty acid-binding protein FABP7, which is expressed in astrocytes and neural progenitors, is a member of the intracellular lipid-binding protein family. This protein is not only involved in various cellular functions such as metabolism, inflammation and energy homeostasis, but also in diseases such as cognitive disorders and tumors. Structures of unsaturated fatty acids, such as oleic acid (OA) and docosahexaenoic acid (DHA), bound to FABP7 have been elucidated; however, structures of saturated fatty acids bound to FABP7 remain unknown. To better understand fatty acid recognition, here the crystal structure of human brain-type fatty acid-binding protein FABP7 complexed with palmitic acid (PA), a saturated fatty acid, is reported at a resolution of 1.6 Å. The PA bound to the fatty acid-binding pocket of FABP7 assumed a U-shaped conformation. The carboxylate moiety of PA interacted with Tyr129, Arg127 and, via a water bridge, with Arg107 and Thr54, whereas its aliphatic chain was stabilized by hydrophobic interactions with Met21, Leu24, Thr30, Thr37, Pro39, Phe58 and Asp77. Structural comparison showed that PA, OA and DHA exhibited unique binding conformations in the fatty acid-binding pocket, stabilized by distinct amino-acid interactions. The binding of PA to FABP7 exhibits a unique binding conformation when compared with other human FABPs (FABP3–FABP5 and FABP8) expressed in other tissues. Based on the crystal and fatty acid structures, it was suggested that PA, which prefers a linear form in nature, required a greater conformational change in its aliphatic chain to bind to the fatty acid-binding pocket in a U-shaped conformation, compared with the cis configurations of OA or DHA. This, together with the length of the aliphatic chain, was considered to be one of the factors determining the binding affinity of PA to FABP7. These results provide a better understanding of fatty acid recognition by FABP7 and expand the knowledge of the binding of PA to FABPs.
中文翻译:

与棕榈酸复合的人脑型脂肪酸结合蛋白FABP7的晶体结构
脑型脂肪酸结合蛋白 FABP7 在星形胶质细胞和神经祖细胞中表达,是细胞内脂质结合蛋白家族的成员。这种蛋白质不仅参与各种细胞功能,如新陈代谢、炎症和能量稳态,还参与认知障碍和肿瘤等疾病。已经阐明了与 FABP7 结合的不饱和脂肪酸的结构,例如油酸 (OA) 和二十二碳六烯酸 (DHA);然而,与 FABP7 结合的饱和脂肪酸的结构仍然未知。为了更好地理解脂肪酸识别,这里报道了与饱和脂肪酸棕榈酸 (PA) 复合的人脑型脂肪酸结合蛋白 FABP7 的晶体结构,分辨率为 1.6 Å。与 FABP7 的脂肪酸结合口袋结合的 PA 呈 U 形构象。PA 的羧酸盐部分与 Tyr129、Arg127 相互作用,并通过水桥与 Arg107 和 Thr54 相互作用,而其脂肪链通过与 Met21、Leu24、Thr30、Thr37、Pro39、Phe58 和 Asp77 的疏水相互作用稳定。结构比较表明 PA、OA 和 DHA 在脂肪酸结合口袋中表现出独特的结合构象,通过不同的氨基酸相互作用稳定下来。与在其他组织中表达的其他人类 FABP(FABP3-FABP5 和 FABP8)相比,PA 与 FABP7 的结合表现出独特的结合构象。根据晶体结构和脂肪酸结构,认为本质上更喜欢线性形式的 PA,OA 或 DHA 的顺式配置。这与脂肪链的长度一起被认为是决定 PA 与 FABP7 结合亲和力的因素之一。这些结果提供了对 FABP7 脂肪酸识别的更好理解,并扩展了 PA 与 FABP 结合的知识。
更新日期:2021-07-02
中文翻译:

与棕榈酸复合的人脑型脂肪酸结合蛋白FABP7的晶体结构
脑型脂肪酸结合蛋白 FABP7 在星形胶质细胞和神经祖细胞中表达,是细胞内脂质结合蛋白家族的成员。这种蛋白质不仅参与各种细胞功能,如新陈代谢、炎症和能量稳态,还参与认知障碍和肿瘤等疾病。已经阐明了与 FABP7 结合的不饱和脂肪酸的结构,例如油酸 (OA) 和二十二碳六烯酸 (DHA);然而,与 FABP7 结合的饱和脂肪酸的结构仍然未知。为了更好地理解脂肪酸识别,这里报道了与饱和脂肪酸棕榈酸 (PA) 复合的人脑型脂肪酸结合蛋白 FABP7 的晶体结构,分辨率为 1.6 Å。与 FABP7 的脂肪酸结合口袋结合的 PA 呈 U 形构象。PA 的羧酸盐部分与 Tyr129、Arg127 相互作用,并通过水桥与 Arg107 和 Thr54 相互作用,而其脂肪链通过与 Met21、Leu24、Thr30、Thr37、Pro39、Phe58 和 Asp77 的疏水相互作用稳定。结构比较表明 PA、OA 和 DHA 在脂肪酸结合口袋中表现出独特的结合构象,通过不同的氨基酸相互作用稳定下来。与在其他组织中表达的其他人类 FABP(FABP3-FABP5 和 FABP8)相比,PA 与 FABP7 的结合表现出独特的结合构象。根据晶体结构和脂肪酸结构,认为本质上更喜欢线性形式的 PA,OA 或 DHA 的顺式配置。这与脂肪链的长度一起被认为是决定 PA 与 FABP7 结合亲和力的因素之一。这些结果提供了对 FABP7 脂肪酸识别的更好理解,并扩展了 PA 与 FABP 结合的知识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号