Journal of Molecular Graphics and Modelling ( IF 2.7 ) Pub Date : 2021-06-29 , DOI: 10.1016/j.jmgm.2021.107974 Melanie Schneider 1 , Jean-Luc Pons 1 , Gilles Labesse 1
|
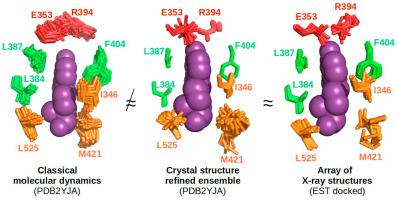
Protein flexibility is challenging for both experimentalists and modellers, especially in the field of drug design. Estrogen Receptor alpha (ERα) is an extensively studied Nuclear Receptor (NR) and a well-known therapeutic target with an important role in development and physiology. It is also a frequent off-target in standard toxicity tests for endocrine disruption. Here, we aim to evaluate the degree to which the conformational space and macromolecular flexibility of this well-characterized drug target can be described. Our approach exploits hundreds of crystallographic structures by means of molecular dynamics simulations and of virtual screening. The analysis of hundreds of crystal structures confirms the presence of two main conformational states, known as ’agonist’ and ’antagonist’, that mainly differ by the orientation of the C-terminal helix H12 which serves to close the binding pocket. ERα also shows some loop flexibility, as well as variable side-chain orientations in its active site. We scrutinized the extent to which standard molecular dynamics simulations or crystallographic refinement as ensemble recapitulate most of the variability features seen by the array of available crystal structures. In parallel, we investigated on the kind and extent of flexibility that are required to achieve convincing docking for all high-affinity ERα ligands present in BindingDB. Using either only one conformation with a few side-chains set flexible, or static structure ensembles in parallel during docking led to good quality and similar pose predictions. These results suggest that the several hundreds of crystal structures already known can properly describe the whole conformational universe of ERα′s ligand binding domain. This opens the road for better drug design and affinity computation.
中文翻译:

探索药物设计受体的构象空间:ERα案例研究
蛋白质的灵活性对实验者和建模者来说都是一个挑战,尤其是在药物设计领域。雌激素受体α(ER α) 是一种广泛研究的核受体 (NR) 和众所周知的治疗靶点,在发育和生理学中具有重要作用。在内分泌干扰的标准毒性测试中,它也是一个常见的脱靶点。在这里,我们的目标是评估可以描述这种充分表征的药物靶标的构象空间和大分子灵活性的程度。我们的方法通过分子动力学模拟和虚拟筛选利用了数百种晶体结构。对数百种晶体结构的分析证实了两种主要构象状态的存在,称为“激动剂”和“拮抗剂”,主要区别在于 C 端螺旋 H12 的方向,用于关闭结合口袋。ER α还显示了一些循环灵活性,以及其活性位点的可变侧链方向。我们仔细研究了标准分子动力学模拟或晶体学细化作为整体概括了可用晶体结构阵列所见的大部分可变性特征的程度。同时,我们研究了 BindingDB 中存在的所有高亲和力 ER α配体实现令人信服的对接所需的灵活性的种类和程度。在对接期间仅使用一种具有一些侧链设置灵活的构象或静态结构集成可以产生良好的质量和类似的姿势预测。这些结果表明,已知的数百种晶体结构可以正确描述 ER 的整个构象宇宙α的配体结合域。这为更好的药物设计和亲和力计算开辟了道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号