Enzyme and Microbial Technology ( IF 3.4 ) Pub Date : 2021-06-29 , DOI: 10.1016/j.enzmictec.2021.109862 Na Gu 1 , Simin Liu 1 , Cong Qiu 1 , Linguo Zhao 1 , Jianjun Pei 1
|
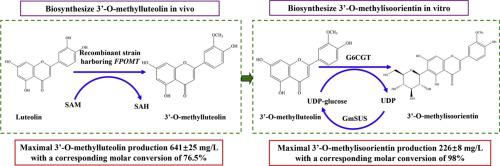
Glycosylation and methylation of flavonoids are the main types of structural modifications and can endow flavonoids with greater stability, bioactivity, and bioavailability. In this study, five types of O-methyltransferases were screened for producing O-methylated luteolin, and the biosynthesis strategy of 3’-O-methylisoorientin from luteolin was determined. To improve the production of 3’-O-methylluteolin, the S-adenosyl-l-methionine synthesis pathway was reconstructed in the recombinant strain by introducing S-adenosyl-l-methionine synthetase genes. After optimizing the conversion conditions, maximal 3’-O-methylluteolin production reached 641 ± 25 mg/L with a corresponding molar conversion of 76.5 %, which was the highest titer of methylated flavonoids reported to date in Escherichia coli. 3’-O-Methylluteolin (127 mg) was prepared from 250 mL of the broth by silica gel column chromatography and preparative HPLC with a yield of 79.4 %. Subsequently, we used the biocatalytic cascade of Gentiana triflora C-glycosyltransferase (Gt6CGT) and Glycine max sucrose synthase (GmSUS) to biosynthesize 3’-O-methylisoorientin from 3’-O-methylluteolin in vitro. By optimizing the coupled reaction conditions and using the fed-batch operation, maximal 3’-O-methylisoorientin production reached 226 ± 8 mg/L with a corresponding molar conversion of 98 %. Therefore, this study provides an efficient method for the production of novel 3’-O-methylisoorientin and the biosynthesis strategy for methylated C-glycosylation flavonoids by selective O-methylation/C-glycosylation motif on flavonoids.
中文翻译:

通过选择 O-甲基化/C-糖基化基序从木犀草素生物合成 3'-O-甲基异定向
黄酮类化合物的糖基化和甲基化是结构修饰的主要类型,可以赋予黄酮类化合物更大的稳定性、生物活性和生物利用度。本研究筛选了5种用于生产O-甲基化木犀草素的O-甲基转移酶,并确定了木犀草素3'- O-甲基异东方素的生物合成策略。为了提高3'- O-甲基木犀草素的产量,通过引入S-腺苷-1-甲硫氨酸合成酶基因在重组菌株中重建了S-腺苷-1-甲硫氨酸合成途径。优化转化条件后,最大3'- O-甲基木犀草素产量达到 641 ± 25 mg/L,相应的摩尔转化率为 76.5%,这是迄今为止在大肠杆菌中报道的甲基化黄酮类化合物的最高滴度。3'- O-甲基木犀草素(127mg)通过硅胶柱色谱法和制备型HPLC从250mL肉汤制备,产率为79.4%。随后,我们使用龙胆草 C-糖基转移酶 (Gt6CGT) 和甘氨酸最大蔗糖合酶 (GmSUS)的生物催化级联反应,在体外从 3'- O-甲基木犀草素生物合成 3'- O-甲基异定向。通过优化偶联反应条件和使用分批补料操作,最大 3'- O-甲基异定向素的产量达到 226 ± 8 mg/L,相应的摩尔转化率为 98%。因此,本研究为生产新型3'- O-甲基异定向素提供了一种有效的方法,并通过黄酮类化合物上的选择性O-甲基化/ C-糖基化基序为甲基化C-糖基化黄酮类化合物提供了生物合成策略。










































 京公网安备 11010802027423号
京公网安备 11010802027423号