当前位置:
X-MOL 学术
›
STEM CELLS
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cytokine and epigenetic regulation of programmed death-ligand 1 in stem cell differentiation and cancer cell plasticity
STEM CELLS ( IF 5.2 ) Pub Date : 2021-06-28 , DOI: 10.1002/stem.3429 Ming-Han Kuo, Pei-Yu Chen, Yi-Ping Yang, Ming-Yi Zheng, Chia-Cheng Miao, Kuo-Chang Wen, Kuo-Ming Chang, Shih-Jie Chou, Mong-Lien Wang, Shih-Hwa Chiou, Yu-Ting Chou
STEM CELLS ( IF 5.2 ) Pub Date : 2021-06-28 , DOI: 10.1002/stem.3429 Ming-Han Kuo, Pei-Yu Chen, Yi-Ping Yang, Ming-Yi Zheng, Chia-Cheng Miao, Kuo-Chang Wen, Kuo-Ming Chang, Shih-Jie Chou, Mong-Lien Wang, Shih-Hwa Chiou, Yu-Ting Chou
|
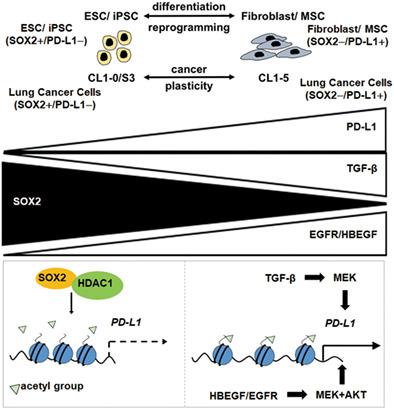
Programmed death-ligand 1 (PD-L1), an immune checkpoint ligand, is recognized as a potential target for cancer immunotherapy as well as for the induction of transplantation tolerance. However, how the crosstalk between stem cell programming and cytokine signaling regulates PD-L1 expression during stem cell differentiation and cancer cell plasticity remains unclear. Herein, we reported that PD-L1 expression was regulated by SOX2 during embryonic stem cell (ESC) differentiation and lung cancer cell plasticity. PD-L1 was induced during ESC differentiation to fibroblasts and was downregulated during SOX2-mediated reprogramming of fibroblasts to induced pluripotent stem cells (iPSCs). Furthermore, SOX2 activation affected cancer cell plasticity and inhibited PD-L1 expression in lung cancer cells. We discovered that the H3K27ac signal at the PD-L1 locus was enhanced during ESC differentiation to fibroblasts as well as during cancer plasticity of SOX2-positive lung cancer cells to SOX2-negative counterparts. Romidepsin, an epigenetic modifier, induced PD-L1 expression in lung cancer cells, whereas TGF-β stimulation downregulated SOX2 but upregulated PD-L1 expression in lung cancer cells. Furthermore, in addition to PD-L1, the expressions of EGFR and its ligand HBEGF were downregulated by activation of endogenous SOX2 expression during lung cancer cell plasticity and iPSC reprogramming, and the activation of EGFR signaling by HBEGF upregulated PD-L1 expression in lung cancer cells. Together, our results reveal the crosstalk between SOX2 programming and cytokine stimulation influences PD-L1 expression, and these findings may provide insights into PD-L1-mediated therapeutics.
中文翻译:

程序性死亡配体1在干细胞分化和癌细胞可塑性中的细胞因子和表观遗传调控
程序性死亡配体 1 (PD-L1) 是一种免疫检查点配体,被认为是癌症免疫治疗以及诱导移植耐受的潜在靶点。然而,干细胞编程和细胞因子信号传导之间的串扰如何在干细胞分化和癌细胞可塑性过程中调节 PD-L1 表达仍不清楚。在此,我们报道了在胚胎干细胞 (ESC) 分化和肺癌细胞可塑性过程中 PD-L1 的表达受 SOX2 的调节。PD-L1 在 ESC 分化为成纤维细胞期间被诱导,并且在 SOX2 介导的成纤维细胞重编程为诱导多能干细胞 (iPSC) 期间被下调。此外,SOX2 激活影响癌细胞的可塑性并抑制肺癌细胞中 PD-L1 的表达。我们发现 H3K27ac 信号在PD-L1基因座在 ESC 分化为成纤维细胞期间以及在 SOX2 阳性肺癌细胞向 SOX2 阴性对应物的癌症可塑性期间增强。罗米地辛是一种表观遗传修饰剂,可诱导肺癌细胞中PD-L1的表达,而 TGF-β 刺激下调SOX2但上调肺癌细胞中的PD-L1表达。此外,除 PD-L1 外,EGFR 及其配体 HBEGF 的表达在肺癌细胞可塑性和 iPSC 重编程过程中通过激活内源性 SOX2 表达而下调,而 HBEGF 激活 EGFR 信号通路上调PD-L1肺癌细胞中的表达。总之,我们的结果揭示了 SOX2 编程和细胞因子刺激之间的串扰影响 PD-L1 表达,这些发现可能为 PD-L1 介导的治疗提供见解。
更新日期:2021-06-28
中文翻译:

程序性死亡配体1在干细胞分化和癌细胞可塑性中的细胞因子和表观遗传调控
程序性死亡配体 1 (PD-L1) 是一种免疫检查点配体,被认为是癌症免疫治疗以及诱导移植耐受的潜在靶点。然而,干细胞编程和细胞因子信号传导之间的串扰如何在干细胞分化和癌细胞可塑性过程中调节 PD-L1 表达仍不清楚。在此,我们报道了在胚胎干细胞 (ESC) 分化和肺癌细胞可塑性过程中 PD-L1 的表达受 SOX2 的调节。PD-L1 在 ESC 分化为成纤维细胞期间被诱导,并且在 SOX2 介导的成纤维细胞重编程为诱导多能干细胞 (iPSC) 期间被下调。此外,SOX2 激活影响癌细胞的可塑性并抑制肺癌细胞中 PD-L1 的表达。我们发现 H3K27ac 信号在PD-L1基因座在 ESC 分化为成纤维细胞期间以及在 SOX2 阳性肺癌细胞向 SOX2 阴性对应物的癌症可塑性期间增强。罗米地辛是一种表观遗传修饰剂,可诱导肺癌细胞中PD-L1的表达,而 TGF-β 刺激下调SOX2但上调肺癌细胞中的PD-L1表达。此外,除 PD-L1 外,EGFR 及其配体 HBEGF 的表达在肺癌细胞可塑性和 iPSC 重编程过程中通过激活内源性 SOX2 表达而下调,而 HBEGF 激活 EGFR 信号通路上调PD-L1肺癌细胞中的表达。总之,我们的结果揭示了 SOX2 编程和细胞因子刺激之间的串扰影响 PD-L1 表达,这些发现可能为 PD-L1 介导的治疗提供见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号