当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Measurement and the Correlation of Cilostazol in Pure and 1,4-Dioxane + Ethanol Binary Solvents from T = 273.15 to 313.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-06-29 , DOI: 10.1021/acs.jced.1c00302 Zhouyu Jiang 1 , Cunbin Du 1 , Yang Cong 1 , Meng Wang 1 , Ke Xing 1 , Yuchao Bian 1 , Xiaoxuan Li 1 , Mingliang Wang 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-06-29 , DOI: 10.1021/acs.jced.1c00302 Zhouyu Jiang 1 , Cunbin Du 1 , Yang Cong 1 , Meng Wang 1 , Ke Xing 1 , Yuchao Bian 1 , Xiaoxuan Li 1 , Mingliang Wang 1
Affiliation
|
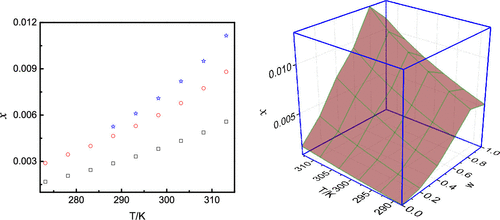
Cilostazol is a neutral molecule with poor solubility. In this work, the solubility data of cilostazol in 10 pure and 1,4-dioxane + ethanol binary solvents was measured using the isothermal saturation method within the temperature range from 273.15 to 313.15 K at atmospheric pressure. In the 10 pure solvents, the results indicated that the maximum and minimum solubility of cilostazol appeared in 1,4-dioxane and isopropanol, respectively. The sequence of solubility was 1,4-dioxane > 2-butanone > acetone > 1-butanol > acetonitrile > methanol > n-propanol > ethanol > ethyl acetate > isopropanol. In a mixture of 1,4-dioxane + ethanol, it first increased and then decreased with increasing mass fraction of 1,4-dioxane in 1,4-dioxane + ethanol mixtures at all selected temperatures. The dissolution process of cilostazol was analyzed by the solvent properties. Moreover, the solubility data of cilostazol in the 10 pure solvents was correlated with the modified Apelblat and λh equations. The result in binary mixture at various temperatures was evaluated by the Jouyban–Acree model. In a pure solvent, the RAD values between the experimental and calculated data with modified Apelblat and λh equations were all no larger than 3.57 × 10–2 and 11.40 × 10–2, respectively. In 1,4-dioxane + ethanol binary mixtures, the RAD data of the Jouyban–Acree model was 6.22 × 10–2. The study of the dissolution process for cilostazol is an important aspect of its purification and recrystallization.
中文翻译:

从T = 273.15 到 313.15 K ,西洛他唑在纯溶剂和 1,4-二恶烷 + 乙醇二元溶剂中的溶解度测量和相关性
西洛他唑是一种溶解性较差的中性分子。在这项工作中,西洛他唑在 10 纯和 1,4-二恶烷 + 乙醇二元溶剂中的溶解度数据使用等温饱和法在 273.15 至 313.15 K 的温度范围内在大气压下测量。结果表明,在10种纯溶剂中,西洛他唑的最大溶解度和最小溶解度分别出现在1,4-二恶烷和异丙醇中。溶解度顺序为1,4-二恶烷>2-丁酮>丙酮>1-丁醇>乙腈>甲醇> n-丙醇>乙醇>乙酸乙酯>异丙醇。在 1,4-二恶烷 + 乙醇的混合物中,在所有选定温度下,随着 1,4-二恶烷 + 乙醇混合物中 1,4-二恶烷的质量分数的增加,它首先增加然后减少。通过溶剂性质分析西洛他唑的溶解过程。此外,西洛他唑在 10 种纯溶剂中的溶解度数据与修改后的 Apelblat 和 λ h方程相关。Jouyban-Acree 模型评估了不同温度下二元混合物的结果。在纯溶剂中,使用修正的 Apelblat 和 λ h方程的实验数据和计算数据之间的 RAD 值均不大于 3.57 × 10 –2和 11.40 × 10 –2, 分别。在 1,4-二恶烷 + 乙醇二元混合物中,Jouyban-Acree 模型的 RAD 数据为 6.22 × 10 –2。西洛他唑溶出过程的研究是其纯化和重结晶的一个重要方面。
更新日期:2021-07-08
中文翻译:

从T = 273.15 到 313.15 K ,西洛他唑在纯溶剂和 1,4-二恶烷 + 乙醇二元溶剂中的溶解度测量和相关性
西洛他唑是一种溶解性较差的中性分子。在这项工作中,西洛他唑在 10 纯和 1,4-二恶烷 + 乙醇二元溶剂中的溶解度数据使用等温饱和法在 273.15 至 313.15 K 的温度范围内在大气压下测量。结果表明,在10种纯溶剂中,西洛他唑的最大溶解度和最小溶解度分别出现在1,4-二恶烷和异丙醇中。溶解度顺序为1,4-二恶烷>2-丁酮>丙酮>1-丁醇>乙腈>甲醇> n-丙醇>乙醇>乙酸乙酯>异丙醇。在 1,4-二恶烷 + 乙醇的混合物中,在所有选定温度下,随着 1,4-二恶烷 + 乙醇混合物中 1,4-二恶烷的质量分数的增加,它首先增加然后减少。通过溶剂性质分析西洛他唑的溶解过程。此外,西洛他唑在 10 种纯溶剂中的溶解度数据与修改后的 Apelblat 和 λ h方程相关。Jouyban-Acree 模型评估了不同温度下二元混合物的结果。在纯溶剂中,使用修正的 Apelblat 和 λ h方程的实验数据和计算数据之间的 RAD 值均不大于 3.57 × 10 –2和 11.40 × 10 –2, 分别。在 1,4-二恶烷 + 乙醇二元混合物中,Jouyban-Acree 模型的 RAD 数据为 6.22 × 10 –2。西洛他唑溶出过程的研究是其纯化和重结晶的一个重要方面。











































 京公网安备 11010802027423号
京公网安备 11010802027423号