当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stability Conditions for Semiclathrate Hydrates Formed with Tetrabutylammonium Chloride + Tetrabutylphosphonium Chloride + CH4
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-06-28 , DOI: 10.1021/acs.jced.1c00309 Lingli Shi 1, 2, 3 , Yong He 1, 2, 3 , Jingsheng Lu 1, 2, 3 , Deqing Liang 1, 2, 3
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-06-28 , DOI: 10.1021/acs.jced.1c00309 Lingli Shi 1, 2, 3 , Yong He 1, 2, 3 , Jingsheng Lu 1, 2, 3 , Deqing Liang 1, 2, 3
Affiliation
|
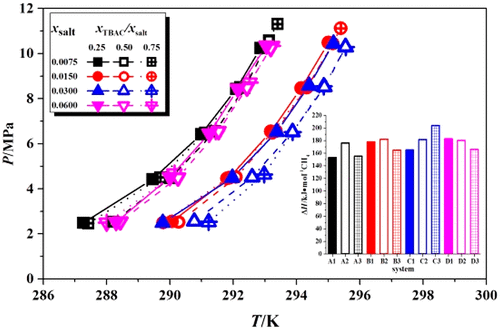
Semiclathrate hydrate is a promising method to ease the thermodynamic conditions of gas hydrate formation for hydrate-based gas storage and transportation on the industrial scale. The phase equilibrium conditions of mixed semiclathrate hydrate formed with tetrabutylammonium chloride (TBAC) + tetrabutylphosphonium chloride (TBPC) + CH4 were measured systematically by employing the isochoric pressure-search method. The data revealed that mixed TBAC + TBPC exerted relatively higher stabilization effect on CH4 hydrate than pure TBAC or TBPC, and the strongest stabilization effect showed up in system with 0.0300 total salt mole fraction (xsalt) and 0.75 TBAC salt ratio (xTBAC/xsalt). In addition, the dissociation enthalpies of mixed (TBAC + TBPC + CH4) hydrate were calculated with the obtained experimental data by using the simplified Clausius–Clapeyron equation and the Peng–Robinson equation of state. It was found that the mean dissociation enthalpies for mixed (TBAC + TBPC + CH4) hydrate with different salt concentration relied on a (153.04 to 203.92 kJ/molCH4) scale, which was significantly higher than that of the pure CH4 hydrate. The data generated in this work showed that mixed TBAC + TBPC could enhance CH4 hydrate stability and the stored energy effectively.
中文翻译:

由四丁基氯化铵 + 四丁基氯化鏻 + CH4 形成的半包合物水合物的稳定性条件
半包合物水合物是缓解天然气水合物形成热力学条件的一种很有前景的方法,可用于工业规模的水合物储运。采用等容压探法系统地测定了四丁基氯化铵(TBAC)+四丁基氯化鏻(TBPC)+CH 4形成的混合半包合物水合物的相平衡条件。数据表明,TBAC + TBPC对CH 4水合物的稳定作用比纯TBAC或TBPC高,在总盐摩尔分数( x salt )为0.0300 ,TBAC盐比( x TBAC )为0.75的体系中稳定作用最强。/ x盐)。此外,混合(TBAC + TBPC + CH 4)水合物的解离焓通过使用简化的Clausius-Clapeyron方程和Peng-Robinson状态方程根据获得的实验数据计算。结果表明,不同盐浓度的混合(TBAC + TBPC + CH 4)水合物的平均解离焓依赖于(153.04~203.92 kJ/molCH 4)尺度,明显高于纯CH 4水合物。这项工作中产生的数据表明,混合TBAC + TBPC 可以有效地提高CH 4水合物的稳定性和储能。
更新日期:2021-06-28
中文翻译:

由四丁基氯化铵 + 四丁基氯化鏻 + CH4 形成的半包合物水合物的稳定性条件
半包合物水合物是缓解天然气水合物形成热力学条件的一种很有前景的方法,可用于工业规模的水合物储运。采用等容压探法系统地测定了四丁基氯化铵(TBAC)+四丁基氯化鏻(TBPC)+CH 4形成的混合半包合物水合物的相平衡条件。数据表明,TBAC + TBPC对CH 4水合物的稳定作用比纯TBAC或TBPC高,在总盐摩尔分数( x salt )为0.0300 ,TBAC盐比( x TBAC )为0.75的体系中稳定作用最强。/ x盐)。此外,混合(TBAC + TBPC + CH 4)水合物的解离焓通过使用简化的Clausius-Clapeyron方程和Peng-Robinson状态方程根据获得的实验数据计算。结果表明,不同盐浓度的混合(TBAC + TBPC + CH 4)水合物的平均解离焓依赖于(153.04~203.92 kJ/molCH 4)尺度,明显高于纯CH 4水合物。这项工作中产生的数据表明,混合TBAC + TBPC 可以有效地提高CH 4水合物的稳定性和储能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号