Primary Care Diabetes ( IF 2.9 ) Pub Date : 2021-06-25 , DOI: 10.1016/j.pcd.2021.06.008 Neda Rajamand Ekberg 1 , Ulrik Bodholdt 2 , Andrei-Mircea Catarig 3 , Sergiu-Bogdan Catrina 1 , Katrine Grau 3 , Cecilia Nagorny Holmberg 3 , Boris Klanger 4 , Søren Tang Knudsen 5
|
Aims
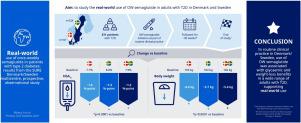
As part of the SURE programme, SURE Denmark/Sweden aimed to study the real-world use of once-weekly (OW) semaglutide in adults with type 2 diabetes (T2D) in Denmark/Sweden.
Methods
SURE Denmark/Sweden was an ∼30-week, prospective, multicentre, open-label, observational study, enrolling adults with T2D and ≥1 documented HbA1c value ≤12 weeks before initiating semaglutide at their physician’s discretion. Primary (change in HbA1c) and secondary (including change in body weight, glycaemic and weight-loss target achievement) endpoints were assessed between baseline and end of study (EOS).
Results
Of the 331 patients initiating semaglutide, 282 (85%) completed the study on treatment. For the latter, estimated mean changes [95% confidence interval] in HbA1c and body weight between baseline and EOS were –1.2 [–1.3; –1.1]%-points (–13 [–14; –12] mmol/mol) and –5.4 [–6.0; –4.7] kg (both p < 0.0001), respectively, with similar results in Denmark and Sweden. At EOS, 67.5% of patients achieved HbA1c <7%; 49.4% achieved a weight reduction of ≥5%. Reported adverse events were consistent with the known safety profile of semaglutide.
Conclusions
In routine clinical practice in Denmark/Sweden, use of OW semaglutide was associated with glycaemic and weight-loss benefits in a wide range of adults with T2D, supporting real-world use.
ClinicalTrials.gov Identifier
NCT03648281.
中文翻译:

每周一次索马鲁肽在 2 型糖尿病患者中的实际应用:SURE 丹麦/瑞典多中心、前瞻性、观察性研究的结果
目标
作为 SURE 计划的一部分,SURE 丹麦/瑞典旨在研究丹麦/瑞典成人 2 型糖尿病 (T2D) 成人每周一次 (OW) semaglutide 的实际使用情况。
方法
SURE Denmark/Sweden 是一项为期 30 周、前瞻性、多中心、开放标签的观察性研究,招募了患有 T2D 且有 ≥1 个记录的 HbA 1c值≤12 周的 T2D 成年人,由医生自行决定开始使用 semaglutide。在基线和研究结束 (EOS) 之间评估主要(HbA 1c的变化)和次要(包括体重、血糖和减肥目标实现的变化)终点。
结果
在开始使用 semaglutide 的 331 名患者中,282 名 (85%) 完成了治疗研究。对于后者,基线和 EOS 之间 HbA 1c和体重的估计平均变化 [95% 置信区间] 为 –1.2 [–1.3;–1.1]% 点 (–13 [–14; –12] mmol/mol) 和 –5.4 [–6.0; –4.7] kg(均 p < 0.0001),丹麦和瑞典的结果相似。在 EOS,67.5% 的患者 HbA 1c <7%;49.4% 实现了 ≥5% 的减重。报告的不良事件与已知的索马鲁肽安全性特征一致。
结论
在丹麦/瑞典的常规临床实践中,OW semaglutide 的使用与广泛的 T2D 成年人的血糖和体重减轻相关,支持现实世界的使用。
ClinicalTrials.gov 标识符
NCT03648281。



























 京公网安备 11010802027423号
京公网安备 11010802027423号