Journal of Pharmaceutical Analysis ( IF 6.1 ) Pub Date : 2021-06-25 , DOI: 10.1016/j.jpha.2021.06.006 Linlin Li 1 , Xinxiang Yu 1 , Dongmin Xie 1 , Ningning Peng 1 , Weilin Wang 1 , Decai Wang 1 , Binglong Li 1
|
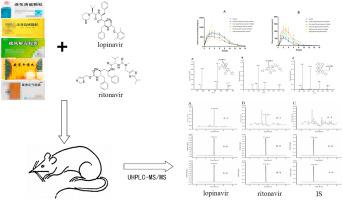
A fast, reliable, and cost-effective liquid chromatography-tandem mass spectrometry method was established to determine the effects of the traditional Chinese medicine employed to treat coronavirus disease 2019, namely, Lianhua Qingwen granules, Huoxiang Zhengqi capsules, Jinhua Qinggan granules, Shufeng Jiedu capsules, and Angong Niuhuang pills, on the pharmacokinetics of lopinavir/ritonavir in rats. Blood samples were prepared using the protein precipitation method and atazanavir was selected as the internal standard (IS). Separation was performed on an Agilent ZORBAX eclipse plus C18 (2.1 mm × 50 mm, 1.8 μm) column using acetonitrile and water containing 0.1% formic acid as the mobile phase for gradient elution. The flow rate was 0.4 mL/min and the injection volume was 2 μL. Agilent Jet Stream electrospray ionization was used for mass spectrometry detection under positive ion multiple reaction monitoring mode at a transition of m/z 629.3→447.3 for lopinavir, m/z 721.3→296.1 for ritonavir, and m/z 705.4→168.1 for the IS. The method showed good linearity in the concentration range of 25–2500 ng/mL (r=0.9981) for lopinavir and 5–500 ng/mL (r=0.9984) for ritonavir. The intra-day and inter-day precision and accuracy were both within ±15%. Items, such as dilution reliability and residual effect, were also within the acceptable limits. The method was used to determine the effects of five types of traditional Chinese medicines on the pharmacokinetics of lopinavir/ritonavir in rats. The pharmacokinetic results showed that the half-life of ritonavir in the groups administered Lianhua Qingwen granules and Huoxiang Zhengqi capsules combined with lopinavir/ritonavir was prolonged by approximately 1.5- to 2-fold relative to that in the control group. Similarly, the pharmacokinetic parameters of lopinavir were altered. Overall, the results of this study offer important theoretical parameters for the effective clinical use of five types of traditional Chinese medicines combined with lopinavir/ritonavir to reduce the occurrence of clinical adverse reactions.
中文翻译:

UHPLC-MS/MS分析中药对洛匹那韦/利托那韦体内代谢的影响
建立了一种快速、可靠、经济高效的液相色谱-串联质谱方法来确定用于治疗2019冠状病毒病的中药连花清瘟颗粒、藿香正气胶囊、金花清肝颗粒、疏风解毒的疗效。胶囊、安宫牛黄丸对洛匹那韦/利托那韦大鼠药代动力学的影响。使用蛋白质沉淀法制备血样,并选择阿扎那韦作为内标(IS)。在 Agilent ZORBAX eclipse plus C 18上进行分离(2.1 mm × 50 mm, 1.8 μm) 色谱柱,使用乙腈和含有 0.1% 甲酸的水作为流动相进行梯度洗脱。流速为 0.4 mL/min,进样量为 2 μL。安捷伦喷射流电喷雾电离用于质谱检测,在正离子多反应监测模式下,洛匹那韦的m/z 629.3→447.3,利托那韦的m/z 721.3→296.1, IS 的m/z 705.4→168.1 . 该方法在 25–2500 ng/mL ( r =0.9981) 的洛匹那韦和 5–500 ng/mL ( r= 0.9984) 用于利托那韦。日内和日间精度和准确度均在±15%以内。稀释可靠性和残留效应等项目也在可接受的范围内。采用该方法测定5种中药对洛匹那韦/利托那韦大鼠药代动力学的影响。药代动力学结果显示,连花清瘟颗粒、藿香正气胶囊联合洛匹那韦/利托那韦组的利托那韦半衰期较对照组延长约1.5~2倍。同样,洛匹那韦的药代动力学参数也发生了改变。总体,











































 京公网安备 11010802027423号
京公网安备 11010802027423号