Insect Biochemistry and Molecular Biology ( IF 3.2 ) Pub Date : 2021-06-25 , DOI: 10.1016/j.ibmb.2021.103610 Yong Hou 1 , Lingzhen Yang 2 , Shuping Xu 2 , Yuhao Zhang 2 , Yuejing Cheng 2 , Yi Li 2 , Jing Gong 3 , Qingyou Xia 1
|
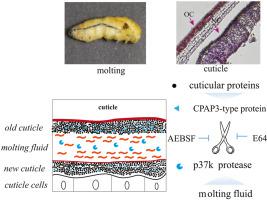
Cuticular proteins analogous to peritrophin 3 (CPAP3)-type cuticle proteins constitute a family of proteins with three chitin-binding domains (CBDs) that play an important role in cuticle formation by associating with chitin. In our previous study, we identified CPAP3-type cuticle proteins in the silkworm genome, of which we characterized CPAP3-A2 (BmCBP1), a protein highly expressed in the epidermis. In this study, to elucidate the digestion mechanism of CPAP3-type cuticle proteins, we incubated CPAP3-A2 with molting fluid in vitro and found that its hydrolysis, which was inhibited by serine and cysteine protease inhibitors, produced two major bands with a molecular weight of approximately 22 kD and 11 kD. A trypsin-type serine protease, p37k, was presumed to be responsible for hydrolyzing CPAP3-A2 based on liquid chromatography-tandem mass spectrometry analysis of naturally purified molting fluid. To verify this, p37k was subsequently expressed in Sf9 cells using the Bac-to-Bac baculovirus expression system. In its active form, the recombinant protease could successfully hydrolyze CPAP3-A2. Finally, we analyzed the CPAP3-A2 molting fluid digestion site. When arginine 169 of CPAP3-A2 was mutated to alanine, a weaker hydrolysis of mutant CPAP3-A2 was observed compared to that of normal CPAP3-A2. Collectively, we identified a trypsin-type serine protease that is involved in the degradation of CPAP3-type cuticle proteins, including CPAP3-A2, suggesting that this protease plays an important role during molting in Bombyx mori. These findings provide the basis for further elucidation of the mechanisms underlying insect molting and metamorphosis.
中文翻译:

胰蛋白酶型丝氨酸蛋白酶 p37k 水解家蚕蜕皮液中的 CPAP3 型角质层蛋白
类似于外周营养蛋白 3 (CPAP3) 型角质层蛋白的角质层蛋白构成一个具有三个几丁质结合域 (CBD) 的蛋白质家族,其通过与几丁质结合在角质层形成中发挥重要作用。在我们之前的研究中,我们在蚕基因组中鉴定了 CPAP3 型角质层蛋白,其中我们表征了 CPAP3-A2 (BmCBP1),一种在表皮中高度表达的蛋白质。在本研究中,为了阐明 CPAP3 型角质层蛋白的消化机制,我们将 CPAP3-A2 与蜕皮液在体外孵育,发现其水解受到丝氨酸和半胱氨酸蛋白酶抑制剂的抑制,产生了两条主要的带分子量约 22 kD 和 11 kD。一种胰蛋白酶型丝氨酸蛋白酶基于对天然纯化蜕皮液的液相色谱-串联质谱分析,推测 p37k 负责水解 CPAP3-A2。为了验证这一点,随后使用Bac-to-Bac杆状病毒表达系统在 Sf9 细胞中表达了 p37k 。在其活性形式下,重组蛋白酶可以成功水解 CPAP3-A2。最后,我们分析了 CPAP3-A2 蜕皮液消化部位。当 CPAP3-A2 的精氨酸 169 突变为丙氨酸时,与正常 CPAP3-A2 的水解相比,观察到突变 CPAP3-A2 的水解较弱。总的来说,我们确定了一种胰蛋白酶型丝氨酸蛋白酶,它参与了 CPAP3 型角质层蛋白(包括 CPAP3-A2)的降解,表明该蛋白酶在蜕皮过程中起着重要作用。家蚕。这些发现为进一步阐明昆虫蜕皮和变态的潜在机制提供了基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号