Journal of Molecular Graphics and Modelling ( IF 2.7 ) Pub Date : 2021-06-26 , DOI: 10.1016/j.jmgm.2021.107979 Nguyen Ngoc Ha 1 , Nguyen Thi Thu Ha 1 , Le Minh Cam 1
|
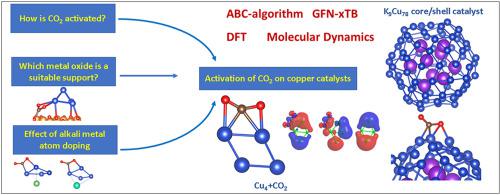
A combination of Artificial Bee Colony algorithm, eXtended Tight Binding and Density functional theory methods were performed to study the activation process of carbon dioxide (CO2) over copper (Cu4 cluster) based catalytic systems. The findings revealed that the activation of the C–O bond resulted from the electron transfer to σ*, π* - MO of CO2. The more the electrons are transferred to CO2, the more the C–O bond is activated and elongated. The suitability of several metal oxide supports (Fe2O3, Al2O3, MgO, ZnO) is estimated using calculated electronic parameters (global electrophilicity index, vertical ionization potential and vertical electron affinity). Aside from demonstrating the appropriateness of Al2O3 and ZnO, a thorough examination of MgO revealed that, due to the formation of stable carbonate products, this oxide is not really appropriate as a support for copper-based catalysts in CO2 conversion. Our studies have also shown that the electron enrichment of copper atoms plays a key role in the activation of C–O bonds. Alkali metal doping (Li, K, Cs) significantly improves the catalytic efficiency of the Cu4 cluster. Based on the results of electron transfer to the CO2 molecule, the effect of doping alkali metal atoms may be organized in the following order: Cs > K > Li. A new core/shell catalytic system with potassium atoms in the core and copper atoms in the shell has been proposed and has proven to be a promising, efficient catalytic system in the CO2 adsorption and activation.
中文翻译:

铜基催化剂上二氧化碳活化机制的新见解:理论研究
进行人工蜂群算法、扩展紧密结合和密度泛函理论方法的组合以研究二氧化碳(CO 2)在基于铜(Cu 4簇)的催化系统上的活化过程。研究结果表明,C-O 键的活化是由电子转移到 CO 2 的σ*, π* - MO 引起的。转移到 CO 2的电子越多,C-O 键被激活和拉长的越多。几种金属氧化物载体(Fe 2 O 3、Al 2 O 3, MgO, ZnO) 使用计算的电子参数(全局亲电性指数、垂直电离势和垂直电子亲和势)进行估计。除了证明 Al 2 O 3和 ZnO的适用性之外,对 MgO 的彻底检查表明,由于形成稳定的碳酸盐产物,这种氧化物并不真正适合作为 CO 2转化中铜基催化剂的载体。我们的研究还表明,铜原子的电子富集在 C-O 键的活化中起着关键作用。碱金属掺杂(Li、K、Cs)显着提高了Cu 4簇的催化效率。基于电子转移到 CO 2 的结果分子中,掺杂碱金属原子的影响可以按以下顺序组织:Cs > K > Li。已经提出了一种新的核/壳催化体系,其核为钾原子,壳为铜原子,并被证明是一种有前途的、高效的 CO 2吸附和活化催化体系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号