Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2021-06-26 , DOI: 10.1007/s10593-021-02964-w Sergey M. Ivanov , Denis S. Koltun
|
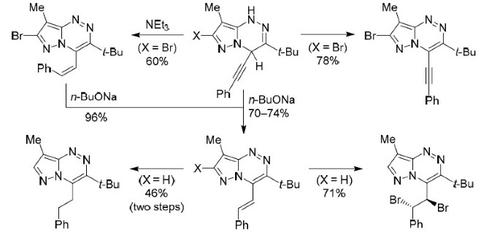
Rearrangements of 3-tert-butyl-8-methyl-4-phenylethynyl-1,4-dihydropyrazolo[5,1-c][1,2,4]triazine derivatives by the action of bases led to the formation of aromatic (E)- or (Z)-4-styryl-functionalized compounds. At the same time, 4-styryl-1,4-dihydropyrazolotriazines did not rearrange to form the expected 4-phenylethylpyrazolo[5,1-c][1,2,4]triazine. The latter was obtained via an alternative route by the addition reaction of phenylethynylmagnesium bromide to 3-tert-butyl-8-methylpyrazolo[5,1-c][1,2,4]triazine, as well as by reduction of double bonds in the ring and side chain of the 4-styryl derivative with subsequent selective oxidation by N-bromosuccinimide. The spectral and X-ray structural data as well as the antimicrobial properties of the synthesized compounds are discussed.
中文翻译:

4-苯基乙炔基和4-苯乙烯基吡唑并[5,1-c][1,2,4]三嗪的合成与转化
3-叔丁基-8-甲基-4-苯基乙炔基-1,4-二氢吡唑并[5,1- c ][1,2,4]三嗪衍生物在碱作用下的重排导致形成芳香族(E )-或( Z )-4-苯乙烯基官能化的化合物。同时,4-苯乙烯基-1,4-二氢吡唑并三嗪没有重排形成预期的4-苯基乙基吡唑并[5,1- c ][1,2,4]三嗪。后者是通过苯乙炔基溴化镁与 3-叔丁基-8-甲基吡唑并[5,1- c][1,2,4]三嗪,以及通过还原 4-苯乙烯基衍生物的环和侧链中的双键,随后通过N-溴代琥珀酰亚胺进行选择性氧化。讨论了合成化合物的光谱和 X 射线结构数据以及抗菌性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号