当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Equilibria in the Quaternary System LiCl–NaCl–SrCl2–H2O at 273 and 323 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-06-24 , DOI: 10.1021/acs.jced.0c01001 Lan-Rong Zhao 1 , Hong-Bao Ren 1 , Shi-Hua Sang 1 , Yun-Yun Gao 1 , Chun-Xia He 1 , Rui-Zhi Cui 2, 3
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-06-24 , DOI: 10.1021/acs.jced.0c01001 Lan-Rong Zhao 1 , Hong-Bao Ren 1 , Shi-Hua Sang 1 , Yun-Yun Gao 1 , Chun-Xia He 1 , Rui-Zhi Cui 2, 3
Affiliation
|
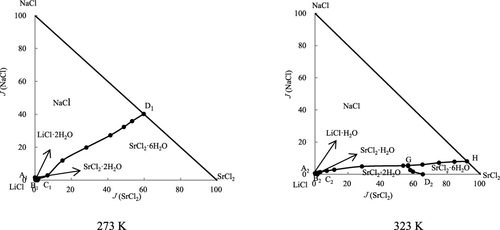
Phase equilibria in the quaternary system LiCl–NaCl–SrCl2–H2O were measured by the isothermal dissolution equilibrium method at 273 and 323 K. The solubility diagrams and water content diagrams of the quaternary system were constructed. The phase diagrams of the quaternary systems LiCl–NaCl–SrCl2–H2O at 273 and 323 K are of the hydrate type and neither double salts nor solid solutions are found. The phase diagram of the quaternary system LiCl–NaCl–SrCl2–H2O at 273 K contains two invariant points, five univariate curves, and four crystallization fields, which correspond to SrCl2·6H2O, SrCl2·2H2O, LiCl·2H2O, and NaCl. The quaternary system LiCl–NaCl–SrCl2–H2O at 323 K has three invariant points, seven univariate curves, and five crystallization fields corresponding to SrCl2·6H2O, SrCl2·2H2O, SrCl2·H2O, LiCl·H2O, and NaCl.
中文翻译:

LiCl-NaCl-SrCl 2 -H 2 O 在 273 和 323 K四元体系中的相平衡
在四元体系 LiCl-NaCl-SrCl 2 -H 2 O 中的相平衡通过等温溶解平衡法在 273 和 323 K 下测量。构建了四元体系的溶解度图和水含量图。四元体系 LiCl-NaCl-SrCl 2 -H 2 O 在 273 和 323 K 的相图是水合物类型,既没有发现复盐也没有发现固溶体。四元体系LiCl-NaCl-SrCl 2 -H 2 O 在273 K的相图包含两个不变点、五个单变量曲线和四个结晶场,分别对应于SrCl 2 ·6H 2 O、SrCl 2 ·2H 2O、LiCl·2H 2 O和NaCl。323 K的四元体系LiCl-NaCl-SrCl 2 -H 2 O具有三个不变点、七个单变量曲线和五个结晶场,分别对应于SrCl 2 ·6H 2 O、SrCl 2 ·2H 2 O、SrCl 2 ·H 2 O、LiCl·H 2 O和NaCl。
更新日期:2021-07-08
中文翻译:

LiCl-NaCl-SrCl 2 -H 2 O 在 273 和 323 K四元体系中的相平衡
在四元体系 LiCl-NaCl-SrCl 2 -H 2 O 中的相平衡通过等温溶解平衡法在 273 和 323 K 下测量。构建了四元体系的溶解度图和水含量图。四元体系 LiCl-NaCl-SrCl 2 -H 2 O 在 273 和 323 K 的相图是水合物类型,既没有发现复盐也没有发现固溶体。四元体系LiCl-NaCl-SrCl 2 -H 2 O 在273 K的相图包含两个不变点、五个单变量曲线和四个结晶场,分别对应于SrCl 2 ·6H 2 O、SrCl 2 ·2H 2O、LiCl·2H 2 O和NaCl。323 K的四元体系LiCl-NaCl-SrCl 2 -H 2 O具有三个不变点、七个单变量曲线和五个结晶场,分别对应于SrCl 2 ·6H 2 O、SrCl 2 ·2H 2 O、SrCl 2 ·H 2 O、LiCl·H 2 O和NaCl。











































 京公网安备 11010802027423号
京公网安备 11010802027423号