Protein & Peptide Letters ( IF 1.0 ) Pub Date : 2021-05-31 , DOI: 10.2174/0929866527666201112122831 Aung,Khine Linn
|
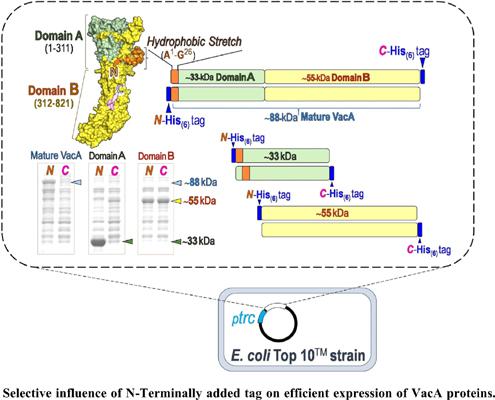
Background: Gastric pathogen Helicobacter pylori secretes VacA cytotoxin displaying a high degree of polymorphic variations of which the highest VacA pathogenicity correlates with m1-type variant followed by VacA-m2.
Objective: To comparatively evaluate expression in Escherichia coli of the mature VacA variants (m1- and m2-types) and their 33- and 55/59-kDa domains fused with His(6) tag at N- or C-terminus.
Methods: All VacA clones expressed in E. coli TOP10™ were analyzed by SDS-PAGE and Western blotting. VacA inclusions were solubilized under native conditions (~150-rpm shaking at 37°C for 2 h in 20 mM HEPES (pH7.4) and 150 mM NaCl). Membrane-perturbing and cytotoxic activities of solubilized VacA proteins were assessed via liposome-entrapped dye leakage and resazurin- based cell viability assays, respectively. VacA binding to human gastric adenocarcinoma cells was assessed by immunofluorescence microscopy. Side-chain hydrophobicity of VacA was analyzed through modeled structures constructed by homology- and ab initio-based modeling.
Results: Both full-length VacA-m1 and 33-kDa domain were efficiently expressed only in the presence of N-terminal extension while its 55-kDa domain was capably expressed with either N- or Cterminal extension. Selectively enhanced expression was also observed for VacA-m2. Protein expression profiles revealed a critical period in IPTG-induced production of the 55-kDa domain with N-terminal extension unlike its C-terminal extension showing relatively stable expression. Both VacA- m1 isolated domains were able to independently bind to cultured gastric cells similar to the full- length toxin, albeit the 33-kDa domain exhibited significantly higher activity of membrane perturbation than others. Membrane-perturbing and cytotoxic activities observed for VacA-m1 appeared to be higher than those of VacA-m2. Homology-based modeling and sequence analysis suggested a potential structural impact of non-polar residues located at the N-terminus of the mature VacA toxin and its 33-kDa domain.
Conclusion: Our data provide molecular insights into selective influence of the N-terminally added tag on efficient expression of recombinant VacA variants, signifying biochemical and biological implications of the hydrophobic stretch within the N-terminal domain.
中文翻译:

N-末端添加的标签选择性增强来自幽门螺杆菌的 VacA 细胞毒素变体的异源表达
背景:胃病原体幽门螺杆菌分泌的 VacA 细胞毒素显示出高度的多态性变异,其中最高的 VacA 致病性与 m1 型变异相关,其次是 VacA-m2。
目的:比较成熟的 VacA 变体(m1 和 m2 型)及其在 N 或 C 端与 His (6)标签融合的 33-kDa 和 55/59-kDa 结构域在大肠杆菌中的表达。
方法:通过 SDS-PAGE 和蛋白质印迹分析在大肠杆菌 TOP10™ 中表达的所有 VacA 克隆。VacA 内含物在天然条件下溶解(在 20 mM HEPES (pH7.4) 和 150 mM NaCl 中,在 37°C 下约 150 rpm 振荡 2 小时)。分别通过脂质体包裹的染料渗漏和基于刃天青的细胞活力测定来评估溶解的 VacA 蛋白的膜扰动和细胞毒性活性。VacA 与人胃腺癌细胞的结合通过免疫荧光显微镜进行评估。VacA 的侧链疏水性通过基于同源性和 ab initio 的建模构建的建模结构进行分析。
结果:全长 VacA-m1 和 33-kDa 结构域仅在存在 N-末端延伸时有效表达,而其 55-kDa 结构域在 N-或 C-末端延伸时能够表达。还观察到 VacA-m2 的选择性增强表达。蛋白质表达谱揭示了 IPTG 诱导产生具有 N 端延伸的 55 kDa 结构域的关键时期,这与其 C 端延伸表现出相对稳定的表达不同。两个 VacA-m1 分离结构域都能够独立地与培养的胃细胞结合,类似于全长毒素,尽管 33-kDa 结构域表现出比其他结构域显着更高的膜扰动活性。对 VacA-m1 观察到的膜扰动和细胞毒性活性似乎高于 VacA-m2。
结论:我们的数据提供了对 N 端添加的标签对重组 VacA 变体有效表达的选择性影响的分子见解,表明 N 端域内疏水性段的生化和生物学意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号