Protein & Peptide Letters ( IF 1.0 ) Pub Date : 2021-05-31 , DOI: 10.2174/0929866527999201126205131 Neelja Singhal 1 , Archana Sharma 1 , Manisha Aswal 1 , Nirpendra Singh 2 , Manish Kumar 1 , Manisha Goel 1
|
Background: CsaA is among the few chaperones which are present in both bacteria and archaea, but absent in eukaryotes. There are no reports on interactome analysis of CsaA from archaea, till date. Identification of binding partners of CsaA might be helpful in understanding CsaA-associated processes in Picrophilus torridus an extreme thermoacidophilic euryarchaeon.
Objectives: The present study was conducted to identify the binding partners of CsaA of P. torridus (PtCsaA).
Methods: The binding partners of PtCsaA were isolated and identified using a pull down assay and liquid chromatography-mass spectrometry (LC-MS).
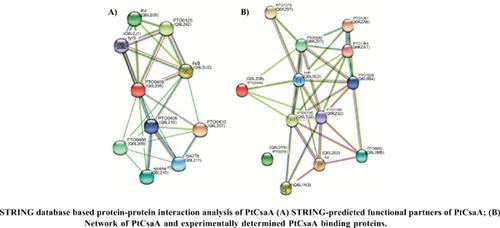
Results: The results revealed twelve potential binding partners of CsaA. These were thermosome subunits (Q6KZS2 and Q6L132), nascent polypeptide-associated complex protein (Q6L1N3), elongation factor 1-alpha (Q6L202), uncharacterized protein (Q6L0Y6), citrate synthase (Q6L0M8), asparaginyl- tRNA synthetase (Q6L0M5), succinyl-CoA synthetase beta chain (Q6L0B4), pyruvate ferredoxin oxidoreductase alpha and beta chain proteins (Q6KZA7 and Q6KZA6, respectively), malate dehydrogenase (Q6L0C3) and reversed fumarylacetoacetase (Q6KZ97). Functional categorization revealed that of these, six proteins were involved in energy metabolic pathways, three were archaeal chaperones, two were involved in translation and one might be a transcription regulator. STRING-based analysis of the protein-protein interactions of the experimental interactome revealed strong interactions among them.
Conclusion: PtCsaA might be a multifaceted protein which besides translation might also play important role in metabolic processes of P. torridus. However, further experiments investigating the binding partners of CsaA in other archaea are required for a better understanding of CsaA-associated processes in archaea.
中文翻译:

CsaA 结合伙伴的鉴定 - 一种Picrophilus torridus的古菌伴侣蛋白
背景:CsaA 是少数存在于细菌和古细菌中但在真核生物中不存在的分子伴侣之一。迄今为止,还没有关于来自古细菌的 CsaA 相互作用组分析的报告。鉴定 CsaA 的结合配偶体可能有助于理解 Picrophilus torridus 中 CsaA 相关过程,这是一种极端嗜热嗜酸的 euryarchaeon。
目的:本研究旨在鉴定 P. torridus (PtCsaA) 的 CsaA 的结合配偶体。
方法:使用下拉测定和液相色谱-质谱法 (LC-MS) 分离和鉴定 PtCsaA 的结合配偶体。
结果:结果揭示了 CsaA 的十二个潜在结合伙伴。这些是嗜热体亚基(Q6KZS2 和 Q6L132)、新生多肽相关复合蛋白(Q6L1N3)、延伸因子 1-α(Q6L202)、未表征的蛋白(Q6L0Y6)、柠檬酸合酶(Q6L0M8)、天冬酰胺酰-tRNA 合成酶(Q6L0琥珀酰化酶) -CoA 合成酶 β 链 (Q6L0B4)、丙酮酸铁氧还蛋白氧化还原酶 α 和 β 链蛋白(分别为 Q6KZA7 和 Q6KZA6)、苹果酸脱氢酶(Q6L0C3)和反向延胡索酰乙酰乙酸酶(Q6KZ97)。功能分类显示,其中6种蛋白质参与能量代谢途径,3种是古菌伴侣蛋白,2种参与翻译,一种可能是转录调节剂。
结论:PtCsaA 可能是一种多方面的蛋白质,除了翻译外,它在 P. torridus 的代谢过程中也可能起重要作用。然而,为了更好地了解古细菌中 CsaA 相关过程,需要进一步研究其他古细菌中 CsaA 的结合伙伴。











































 京公网安备 11010802027423号
京公网安备 11010802027423号