Protein & Peptide Letters ( IF 1.6 ) Pub Date : 2021-05-31 , DOI: 10.2174/0929866527666201112123714 Guo-Ying Qian 1 , Gyutae Lim 2 , Shang-Jun Yin 1 , Jun-Mo Yang 3 , Jinhyuk Lee 2 , Yong-Doo Park 1
|
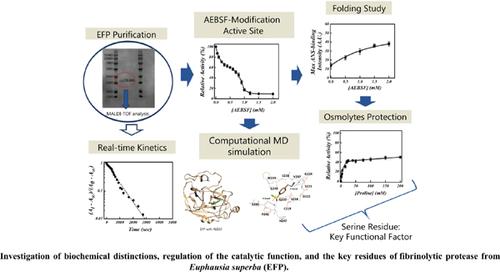
Background: Fibrinolytic protease from Euphausia superba (EFP) was isolated.
Objective: Biochemical distinctions, regulation of the catalytic function, and the key residues of EFP were investigated.
Methods: The serial inhibition kinetic evaluations coupled with measurements of fluorescence spectra in the presence of 4-(2-aminoethyl) benzene sulfonyl fluoride hydrochloride (AEBSF) was conducted. The computational molecular dynamics (MD) simulations were also applied for a comparative study.
Results: The enzyme behaved as a monomeric protein with a molecular mass of about 28.6 kD with Km BApNA = 0.629 ± 0.02 mM and kcat/Km BApNA = 7.08 s-1/mM. The real-time interval measurements revealed that the inactivation was a first-order reaction, with the kinetic processes shifting from a monophase to a biphase. Measurements of fluorescence spectra showed that serine residue modification by AEBSF directly caused conspicuous changes of the tertiary structures and exposed hydrophobic surfaces. Some osmolytes were applied to find protective roles. These results confirmed that the active region of EFP is more flexible than the overall enzyme molecule and serine, as the key residue, is associated with the regional unfolding of EFP in addition to its catalytic role. The MD simulations were supportive to the kinetics data.
Conclusion: Our study indicated that EFP has an essential serine residue for its catalyst function and associated folding behaviors. Also, the functional role of osmolytes such as proline and glycine that may play a role in defense mechanisms from environmental adaptation in a krill’s body was suggested.
中文翻译:

具有多功能丝氨酸蛋白酶活性的磷虾纤溶蛋白酶的生化研究
背景:分离了来自超级磷虾 (EFP) 的纤溶蛋白酶。
目的:研究 EFP 的生化特性、催化功能的调控和关键残基。
方法:在 4-(2-氨基乙基) 苯磺酰氟盐酸盐 (AEBSF) 存在下,进行系列抑制动力学评估和荧光光谱测量。计算分子动力学 (MD) 模拟也用于比较研究。
结果:该酶表现为单体蛋白质,分子量约为 28.6 kD,K m BApNA = 0.629 ± 0.02 mM 和 k cat /K m BApNA= 7.08 s-1/mM。实时间隔测量显示失活是一级反应,动力学过程从单相转变为双相。荧光光谱测量表明,AEBSF 对丝氨酸残基的修饰直接引起三级结构的显着变化和暴露的疏水表面。一些渗透剂被用于寻找保护作用。这些结果证实,EFP 的活性区域比整个酶分子更灵活,丝氨酸作为关键残基,除了具有催化作用外,还与 EFP 的区域解折叠有关。MD 模拟支持动力学数据。
结论:我们的研究表明,EFP 具有对其催化功能和相关折叠行为必不可少的丝氨酸残基。此外,有人提出了渗透质的功能作用,如脯氨酸和甘氨酸,它们可能在磷虾体内环境适应的防御机制中发挥作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号