Nano Research ( IF 9.5 ) Pub Date : 2021-06-25 , DOI: 10.1007/s12274-021-3574-x Sijin Xiang , Zhongxiong Fan , Zichen Ye , Tianbao Zhu , Dao Shi , Shefang Ye , Zhenqing Hou , Xiaolan Chen
|
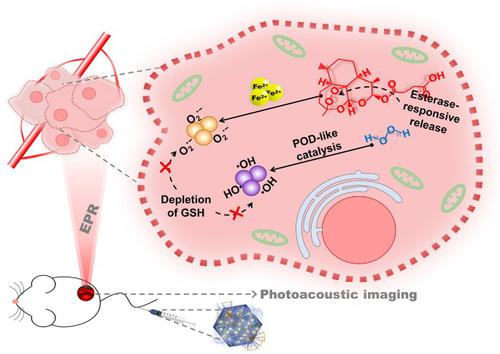
Chemodynamic therapy (CDT) is well acknowledged as potent reactive oxygen species (ROS)-mediated anticancer strategy. Especially, the study about labile iron pool (LIP) as endogenous ferrous catalyzer has paved the way for future CDT development. However, limited H2O2 expression, mild acidity, reduced glutathione (GSH) ablation of ROS, etc., all require employing alternate peroxo-complex to achieve enhanced CDT effect. As a non-Fenton-type substrate, artesunate (ART) has been utilized as a source of free radicals through decomposition of endoperoxide bridges catalyzed by ferrous ions, nonetheless, the non-tumor-specific delivery, inferior pharmacokinetics, and hydrophobic nature minimize the efficacy of ART in physiological systems. Herein, we devise a PPA nanoamplifier by conjugating ART with PEG-functionalized Pd@Pt nanoplates (PP NPs) to form ester linkage, ensuring specific intratumoral esterase-responsive ART release. Significantly, the PPA nanoamplifier combines the in situ decomposition of ART’s endoperoxide bridges by Fe2+ to superoxide anions (O2·−) and peroxidase (POD)-like enzymatic catalysis of endogenous H2O2 by PP to hydroxyl radicals (·OH), thus achieving amplified ROS-mediated tumor therapy. Besides, PPA displays GSH destruction potential, thereby protecting ROS from the cleavage by GSH oxidation. In addition, the strong absorption of PPA in near-infrared (NIR) region also endows PPA with photoacoustic property to realize imaging-guided CDT. In short, by taking advantages of the high enrichment and esterase- responsive drug release at tumor sites, PPA amplified ROS signals via dual pathways, killing tumor cells in vitro and inhibiting tumor growth in vivo, thereby realizing high-efficiency non-Fenton CDT. We believe our novel anti-tumor strategy based on PPA will broaden the future of ROS-mediated tumor-targeted therapy.
中文翻译:

内源性 Fe2+ 激活的 ROS 纳米放大器用于酯酶响应和光声成像监测的治疗改进
化学动力学疗法 (CDT) 被公认为有效的活性氧 (ROS) 介导的抗癌策略。尤其是不稳定铁池(LIP)作为内源性亚铁催化剂的研究为未来CDT的发展铺平了道路。然而,有限的 H 2 O 2表达、温和的酸性、ROS 的还原型谷胱甘肽 (GSH) 消融等,都需要采用交替的过氧复合物来实现增强的 CDT 效果。作为非芬顿型底物,青蒿琥酯 (ART) 已被用作通过亚铁离子催化分解内过氧化物桥的自由基来源,然而,非肿瘤特异性递送、较差的药代动力学和疏水性最大限度地减少了自由基的产生。 ART 在生理系统中的功效。在此,我们通过将 ART 与 PEG 功能化的 Pd@Pt 纳米板 (PP NPs) 结合形成酯键来设计 PPA 纳米放大器,确保特定的肿瘤内酯酶响应性 ART 释放。值得注意的是,PPA 纳米放大器结合了Fe 2+对 ART 内过氧化物桥的原位分解超氧阴离子(O 2 ·-)和过氧化物酶(POD)样的内源性H 2 O 2酶催化PP 生成羟基自由基(·OH),从而实现放大的ROS 介导的肿瘤治疗。此外,PPA 显示出 GSH 破坏潜力,从而保护 ROS 免受 GSH 氧化的裂解。此外,PPA在近红外(NIR)区域的强吸收也赋予了PPA光声特性以实现成像引导的CDT。总之,PPA利用肿瘤部位高富集和酯酶反应性药物释放的优势,通过双重途径放大ROS信号,体外杀伤肿瘤细胞,体内抑制肿瘤生长。,从而实现高效的非芬顿CDT。我们相信我们基于 PPA 的新型抗肿瘤策略将拓宽 ROS 介导的肿瘤靶向治疗的未来。











































 京公网安备 11010802027423号
京公网安备 11010802027423号