Nanomedicine: Nanotechnology, Biology and Medicine ( IF 4.2 ) Pub Date : 2021-06-24 , DOI: 10.1016/j.nano.2021.102418 Renata F Saito 1 , Maria Cristina Rangel 1 , Justin R Halman 2 , Morgan Chandler 2 , Luciana Nogueira de Sousa Andrade 1 , Silvina Odete-Bustos 1 , Tatiane Katsue Furuya 1 , Alexis Germán Murillo Carrasco 1 , Adriano B Chaves-Filho 3 , Marcos Y Yoshinaga 3 , Sayuri Miyamoto 3 , Kirill A Afonin 2 , Roger Chammas 4
|
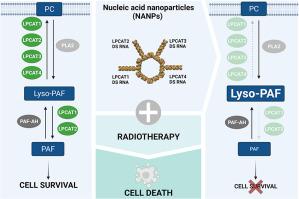
Radiation induces the generation of platelet-activating factor receptor (PAF-R) ligands, including PAF and oxidized phospholipids. Alternatively, PAF is also synthesized by the biosynthetic enzymes lysophosphatidylcholine acyltransferases (LPCATs) which are expressed by tumor cells including melanoma. The activation of PAF-R by PAF and oxidized lipids triggers a survival response protecting tumor cells from radiation-induced cell death, suggesting the involvement of the PAF/PAF-R axis in radioresistance. Here, we investigated the role of LPCATs in the melanoma cell radiotherapy response. LPCAT is a family of four enzymes, LPCAT1-4, and modular nucleic acid nanoparticles (NANPs) allowed for the simultaneous silencing of all four LPCATs. We found that the in vitro simultaneous silencing of all four LPCAT transcripts by NANPs enhanced the therapeutic effects of radiation in melanoma cells by increasing cell death, reducing long-term cell survival, and activating apoptosis. Thus, we propose that NANPs are an effective strategy for improving radiotherapy efficacy in melanomas.
中文翻译:

核酸纳米粒子 (NANPs) 同时沉默溶血磷脂酰胆碱酰基转移酶 1-4 可改善黑色素瘤细胞的辐射反应
辐射诱导血小板活化因子受体 (PAF-R) 配体的产生,包括 PAF 和氧化磷脂。或者,PAF 也由生物合成酶溶血磷脂酰胆碱酰基转移酶 (LPCAT) 合成,该酶由包括黑色素瘤在内的肿瘤细胞表达。PAF 和氧化脂质对 PAF-R 的激活触发了保护肿瘤细胞免受辐射诱导的细胞死亡的存活反应,这表明 PAF/PAF-R 轴参与了辐射抗性。在这里,我们研究了 LPCAT 在黑色素瘤细胞放射治疗反应中的作用。LPCAT 是一种由四种酶 LPCAT1-4 和模块化核酸纳米颗粒 (NANP) 组成的家族,可同时沉默所有四种LPCAT。我们发现体外NANPs同时沉默所有四种LPCAT转录物通过增加细胞死亡、降低长期细胞存活和激活细胞凋亡来增强辐射对黑色素瘤细胞的治疗效果。因此,我们提出 NANPs 是提高黑色素瘤放疗疗效的有效策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号