当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A novel self-targeting theranostic nanoplatform for photoacoustic imaging-monitored and enhanced chemo-sonodynamic therapy
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2021-06-10 , DOI: 10.1039/d1tb01025e Yifan Yang 1 , Zhongxiong Fan 1 , Kaili Zheng 2 , Dao Shi 1 , Guanghao Su 3 , Dongtao Ge 1 , Qingliang Zhao 2 , Xu Fu 4 , Zhenqing Hou 1
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2021-06-10 , DOI: 10.1039/d1tb01025e Yifan Yang 1 , Zhongxiong Fan 1 , Kaili Zheng 2 , Dao Shi 1 , Guanghao Su 3 , Dongtao Ge 1 , Qingliang Zhao 2 , Xu Fu 4 , Zhenqing Hou 1
Affiliation
|
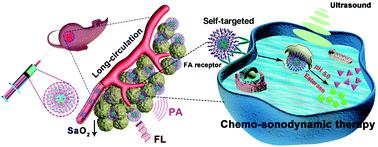
Sonodynamic therapy has attracted wide attention as a noninvasive therapy due to deep tissue penetration. However, majority sonosensitizers often suffer from poor physiological stability, rapid blood clearance and nonspecific targeting, which seriously hinders their further practical applications. Inspired by the concept of active targeting drug delivery, both dual-functional chemo-drug pemetrexed (PEM, emerges an innate affinity toward the folate receptor) and amphiphilic D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) were selected to be covalently linked by an esterase-responsive ester linkage. The synthesized self-targeting TPGS–PEM prodrug and indocyanine green (ICG) as functional motifs can be self-assembled into a TPGS–PEM–ICG nanoplatform within an aqueous medium. The TPGS–PEM–ICG nanoplatform with outstanding structural and physiological stability not only protects the sonosensitizer from reticular endothelial system clearance but also achieves active targeting drug delivery and efficient tumor enrichment. Moreover, TPGS–PEM–ICG nanoplatform can selectively recognize tumor cells and then realize on-demand drug burst release by multiple stimuli of internal lysosomal acidity, esterase and external ultrasound, which guarantee low side effects toward normal tissues and organs. It is also worth noting that our nanoplatform exhibits protruding tumor enrichment under the precise guidance of photoacoustic/fluorescence imaging. Further in vitro and in vivo experimental results well confirmed that the TPGS–PEM–ICG nanoplatform possesses enhanced chemo-sonodynamic effects. Interestingly, the highly toxic reactive oxygen species can remarkably reduce the blood oxygen saturation signal of the tumor microenvironment via precise, multifunctional and high-resolution photoacoustic imaging. Taken together, the TPGS–PEM–ICG nanoplatform can be expected to hold enormous potential for diagnosis, prognosis and targeted therapy for tumor.
中文翻译:

一种用于光声成像监测和增强化学声动力学治疗的新型自靶向治疗纳米平台
由于深层组织穿透,声动力疗法作为一种无创疗法引起了广泛关注。然而,大多数声敏剂往往存在生理稳定性差、血液清除速度快和非特异性靶向等问题,严重阻碍了其进一步的实际应用。受主动靶向给药概念的启发,双功能化学药物培美曲塞(PEM,对叶酸受体具有先天亲和力)和两亲性D-α-生育酚聚乙二醇 1000 琥珀酸酯 (TPGS) 被选择为通过酯酶响应性酯键共价连接。合成的自靶向 TPGS-PEM 前药和吲哚菁绿 (ICG) 作为功能基序可以在水性介质中自组装成 TPGS-PEM-ICG 纳米平台。具有出色结构和生理稳定性的 TPGS-PEM-ICG 纳米平台不仅可以保护声敏剂免受网状内皮系统的清除,还可以实现主动靶向药物递送和有效的肿瘤富集。此外,TPGS-PEM-ICG纳米平台可以选择性识别肿瘤细胞,通过内部溶酶体酸度、酯酶和外部超声的多重刺激实现按需药物突释,保证了对正常组织器官的低副作用。还值得注意的是,我们的纳米平台在光声/荧光成像的精确引导下表现出突出的肿瘤富集。更远体外和体内实验结果很好地证实了 TPGS-PEM-ICG 纳米平台具有增强的化学声动力学效应。有趣的是,剧毒的活性氧可以通过精确、多功能和高分辨率的光声成像显着降低肿瘤微环境的血氧饱和度信号。总之,TPGS-PEM-ICG纳米平台有望在肿瘤的诊断、预后和靶向治疗方面具有巨大的潜力。
更新日期:2021-06-24
中文翻译:

一种用于光声成像监测和增强化学声动力学治疗的新型自靶向治疗纳米平台
由于深层组织穿透,声动力疗法作为一种无创疗法引起了广泛关注。然而,大多数声敏剂往往存在生理稳定性差、血液清除速度快和非特异性靶向等问题,严重阻碍了其进一步的实际应用。受主动靶向给药概念的启发,双功能化学药物培美曲塞(PEM,对叶酸受体具有先天亲和力)和两亲性D-α-生育酚聚乙二醇 1000 琥珀酸酯 (TPGS) 被选择为通过酯酶响应性酯键共价连接。合成的自靶向 TPGS-PEM 前药和吲哚菁绿 (ICG) 作为功能基序可以在水性介质中自组装成 TPGS-PEM-ICG 纳米平台。具有出色结构和生理稳定性的 TPGS-PEM-ICG 纳米平台不仅可以保护声敏剂免受网状内皮系统的清除,还可以实现主动靶向药物递送和有效的肿瘤富集。此外,TPGS-PEM-ICG纳米平台可以选择性识别肿瘤细胞,通过内部溶酶体酸度、酯酶和外部超声的多重刺激实现按需药物突释,保证了对正常组织器官的低副作用。还值得注意的是,我们的纳米平台在光声/荧光成像的精确引导下表现出突出的肿瘤富集。更远体外和体内实验结果很好地证实了 TPGS-PEM-ICG 纳米平台具有增强的化学声动力学效应。有趣的是,剧毒的活性氧可以通过精确、多功能和高分辨率的光声成像显着降低肿瘤微环境的血氧饱和度信号。总之,TPGS-PEM-ICG纳米平台有望在肿瘤的诊断、预后和靶向治疗方面具有巨大的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号