当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A low-charge-overpotential lithium-CO2 cell based on a binary molten salt electrolyte
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2021-5-27 , DOI: 10.1039/d1ee00068c Di Wang 1, 2, 3, 4, 5 , Jingui Yang 1, 2, 3, 4, 5 , Ping He 1, 2, 3, 4, 5 , Haoshen Zhou 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2021-5-27 , DOI: 10.1039/d1ee00068c Di Wang 1, 2, 3, 4, 5 , Jingui Yang 1, 2, 3, 4, 5 , Ping He 1, 2, 3, 4, 5 , Haoshen Zhou 1, 2, 3, 4, 5
Affiliation
|
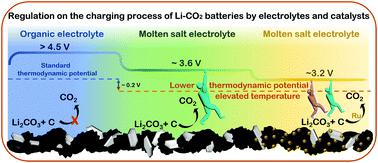
The high charge overpotential of Li–CO2 batteries leads to short calendar life and low energy efficiency. Solid catalysts such as functional carbon materials and noble metals have been studied extensively to facilitate the decomposition of Li2CO3. However, the charge potential is still over 4 V and side reactions of electrolytes are still unavoidable. Unlike progressively developed catalysts, few efforts have been made for the optimization of electrolytes, which is the bottleneck to develop practical Li–CO2 batteries. Here, we demonstrate a binary molten salt Li–CO2 battery operating at elevated temperature. In the presence of an involatile molten salt electrolyte, CO2 evolution during the charging process can be controlled below 3.6 V with a carbon cathode, which is even lower than previously reported values with noble metal catalysts. In addition to this optimized electrolyte solution, Ru nanoparticles with high catalytic activities are also prepared for the solid cathode. Based on this heterogeneous electrocatalysis, the charge potential of this Li–CO2 battery is further reduced to 3.2 V, and the cycling life is extended to 70 cycles. This work provides a promising strategy to extend the working conditions of Li–CO2 batteries to higher temperatures. It also provides a direct insight into the reduction of the high charge overpotential of Li–CO2 batteries, and mitigation of electrolyte decomposition.
中文翻译:

基于二元熔盐电解质的低充电过电位锂二氧化碳电池
Li-CO 2电池的高充电过电位导致日历寿命短和能源效率低。固体催化剂如功能性碳材料和贵金属已被广泛研究以促进 Li 2 CO 3的分解。然而,充电电位仍超过 4 V,电解质的副反应仍然不可避免。与逐步开发的催化剂不同,电解质优化方面的努力很少,这是开发实用 Li-CO 2电池的瓶颈。在这里,我们展示了在高温下运行的二元熔盐 Li-CO 2电池。在不挥发的熔盐电解质存在下,CO 2使用碳阴极可以将充电过程中的演变控制在 3.6 V 以下,这甚至低于之前使用贵金属催化剂报告的值。除了这种优化的电解质溶液外,还为固体阴极制备了具有高催化活性的 Ru 纳米粒子。基于这种非均相电催化,该Li-CO 2电池的充电电位进一步降低至3.2 V,循环寿命延长至70次。这项工作提供了一种有前景的策略,可以将 Li-CO 2电池的工作条件扩展到更高的温度。它还提供了对降低 Li-CO 2电池的高充电过电位和减轻电解质分解的直接见解。
更新日期:2021-06-22
中文翻译:

基于二元熔盐电解质的低充电过电位锂二氧化碳电池
Li-CO 2电池的高充电过电位导致日历寿命短和能源效率低。固体催化剂如功能性碳材料和贵金属已被广泛研究以促进 Li 2 CO 3的分解。然而,充电电位仍超过 4 V,电解质的副反应仍然不可避免。与逐步开发的催化剂不同,电解质优化方面的努力很少,这是开发实用 Li-CO 2电池的瓶颈。在这里,我们展示了在高温下运行的二元熔盐 Li-CO 2电池。在不挥发的熔盐电解质存在下,CO 2使用碳阴极可以将充电过程中的演变控制在 3.6 V 以下,这甚至低于之前使用贵金属催化剂报告的值。除了这种优化的电解质溶液外,还为固体阴极制备了具有高催化活性的 Ru 纳米粒子。基于这种非均相电催化,该Li-CO 2电池的充电电位进一步降低至3.2 V,循环寿命延长至70次。这项工作提供了一种有前景的策略,可以将 Li-CO 2电池的工作条件扩展到更高的温度。它还提供了对降低 Li-CO 2电池的高充电过电位和减轻电解质分解的直接见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号