当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Growth Mechanisms and Phase Equilibria of Propane and Methane + Propane Hydrates
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-06-22 , DOI: 10.1021/acs.jced.1c00329 Sebastian Ovalle 1 , Juan G. Beltran 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-06-22 , DOI: 10.1021/acs.jced.1c00329 Sebastian Ovalle 1 , Juan G. Beltran 1
Affiliation
|
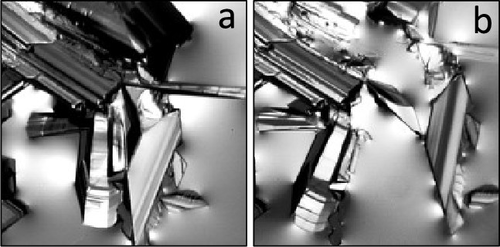
Gas hydrates of light hydrocarbons exhibit complex phase behavior, polymorphism, and diverse crystal morphology. We investigated phase equilibria and growth mechanisms of propane and methane + propane gas hydrates in the range of 0.25 to 2.1 MPa and 273.6 to 287.25 K. Clathrates formed from pure propane and the yCH4 = 0.90 methane + propane mixture exhibited similar morphologies at low subcoolings but differed considerably at higher subcoolings. The yCH4 = 0.98 methane + propane mixture formed crystals with radically different morphologies than those of pure propane and the yCH4 = 0.90 mixture. We report a new hydrate growth mechanism that proceeds via partial dissociation of the growing crystal in propane and propane + methane hydrates.
中文翻译:

丙烷和甲烷 + 丙烷水合物的生长机制和相平衡
轻烃的气体水合物表现出复杂的相行为、多晶型和不同的晶体形态。我们研究了 0.25 至 2.1 MPa 和 273.6 至 287.25 K 范围内的丙烷和甲烷 + 丙烷气体水合物的相平衡和生长机制。由纯丙烷和y CH 4 = 0.90 甲烷 + 丙烷混合物形成的包合物在低温下表现出相似的形态过冷度,但在较高过冷度下差异很大。所述ÿ CH 4 = 0.98 +甲烷丙烷混合物用完全不同的形态比纯丙烷和形成的晶体ÿ CH 4= 0.90 混合物。我们报告了一种新的水合物生长机制,该机制通过丙烷和丙烷 + 甲烷水合物中生长的晶体的部分解离进行。
更新日期:2021-06-22
中文翻译:

丙烷和甲烷 + 丙烷水合物的生长机制和相平衡
轻烃的气体水合物表现出复杂的相行为、多晶型和不同的晶体形态。我们研究了 0.25 至 2.1 MPa 和 273.6 至 287.25 K 范围内的丙烷和甲烷 + 丙烷气体水合物的相平衡和生长机制。由纯丙烷和y CH 4 = 0.90 甲烷 + 丙烷混合物形成的包合物在低温下表现出相似的形态过冷度,但在较高过冷度下差异很大。所述ÿ CH 4 = 0.98 +甲烷丙烷混合物用完全不同的形态比纯丙烷和形成的晶体ÿ CH 4= 0.90 混合物。我们报告了一种新的水合物生长机制,该机制通过丙烷和丙烷 + 甲烷水合物中生长的晶体的部分解离进行。











































 京公网安备 11010802027423号
京公网安备 11010802027423号