当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Structure-Based Design of SARS-CoV-2 nsp14 Methyltransferase Ligands Yields Nanomolar Inhibitors
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2021-06-21 , DOI: 10.1021/acsinfecdis.1c00131 Tomáš Otava 1, 2 , Michal Šála 1 , Fengling Li , Jindřich Fanfrlík 1 , Kanchan Devkota , Sumera Perveen , Irene Chau , Paknoosh Pakarian , Pavel Hobza 1 , Masoud Vedadi , Evzen Boura 1 , Radim Nencka 1
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2021-06-21 , DOI: 10.1021/acsinfecdis.1c00131 Tomáš Otava 1, 2 , Michal Šála 1 , Fengling Li , Jindřich Fanfrlík 1 , Kanchan Devkota , Sumera Perveen , Irene Chau , Paknoosh Pakarian , Pavel Hobza 1 , Masoud Vedadi , Evzen Boura 1 , Radim Nencka 1
Affiliation
|
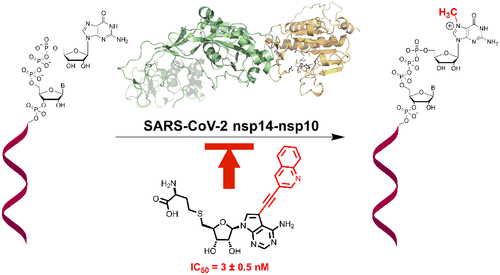
In this study, we have focused on the structure-based design of the inhibitors of one of the two SARS-CoV-2 methyltransferases (MTases), nsp14. This MTase catalyzes the transfer of the methyl group from S-adenosyl-l-methionine (SAM) to cap the guanosine triphosphate moiety of the newly synthesized viral RNA, yielding the methylated capped RNA and S-adenosyl-l-homocysteine (SAH). As the crystal structure of SARS-CoV-2 nsp14 is unknown, we have taken advantage of its high homology to SARS-CoV nsp14 and prepared its homology model, which has allowed us to identify novel SAH derivatives modified at the adenine nucleobase as inhibitors of this important viral target. We have synthesized and tested the designed compounds in vitro and shown that these derivatives exert unprecedented inhibitory activity against this crucial enzyme. The docking studies nicely explain the contribution of an aromatic part attached by a linker to the position 7 of the 7-deaza analogues of SAH.
中文翻译:

SARS-CoV-2 nsp14 甲基转移酶配体基于结构的设计产生纳摩尔抑制剂
在这项研究中,我们重点关注两种 SARS-CoV-2 甲基转移酶 (MTase) 之一 nsp14 的抑制剂的基于结构的设计。该 MTase 催化甲基从S-腺苷-l-甲硫氨酸 (SAM) 转移,以对新合成的病毒 RNA 的三磷酸鸟苷部分进行加帽,产生甲基化加帽 RNA 和S-腺苷-l-高半胱氨酸 (SAH)。由于SARS-CoV-2 nsp14的晶体结构未知,我们利用其与SARS-CoV nsp14的高度同源性,建立了其同源模型,这使我们能够鉴定出腺嘌呤核碱基修饰的新型SAH衍生物作为抑制剂这个重要的病毒目标。我们在体外合成并测试了所设计的化合物,并表明这些衍生物对这种关键酶具有前所未有的抑制活性。对接研究很好地解释了由连接子连接的芳香部分对 SAH 7-脱氮类似物的 7 位的贡献。
更新日期:2021-08-13
中文翻译:

SARS-CoV-2 nsp14 甲基转移酶配体基于结构的设计产生纳摩尔抑制剂
在这项研究中,我们重点关注两种 SARS-CoV-2 甲基转移酶 (MTase) 之一 nsp14 的抑制剂的基于结构的设计。该 MTase 催化甲基从S-腺苷-l-甲硫氨酸 (SAM) 转移,以对新合成的病毒 RNA 的三磷酸鸟苷部分进行加帽,产生甲基化加帽 RNA 和S-腺苷-l-高半胱氨酸 (SAH)。由于SARS-CoV-2 nsp14的晶体结构未知,我们利用其与SARS-CoV nsp14的高度同源性,建立了其同源模型,这使我们能够鉴定出腺嘌呤核碱基修饰的新型SAH衍生物作为抑制剂这个重要的病毒目标。我们在体外合成并测试了所设计的化合物,并表明这些衍生物对这种关键酶具有前所未有的抑制活性。对接研究很好地解释了由连接子连接的芳香部分对 SAH 7-脱氮类似物的 7 位的贡献。











































 京公网安备 11010802027423号
京公网安备 11010802027423号