Materials Today Energy ( IF 9.0 ) Pub Date : 2021-06-21 , DOI: 10.1016/j.mtener.2021.100802 Teng-Yue Hu , Yamin Zhang , Bi-Shan Lu , Yi-Fan Ma , Yan-Nan Zhu , Ya-Ting Wang , Bo-Yang Zhang , Ze-Qi Zhang , Jian Wang , Yang Yang , Hao-Li Zhang
|
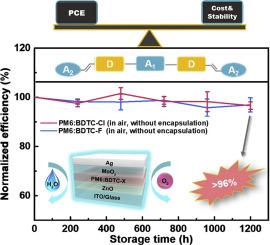
Organic solar cells (OSCs) have made enormous progress in recent years. However, OSCs still suffer from the low price–quality ratio since the complex synthesis of photovoltaic materials and the poor stability of the devices. Comparing with traditional large conjugated ladder-type non-fullerene acceptors, the unfused-ring acceptors with non-covalent intramolecular interactions could notably simplify the synthetic routes while maintaining the device performance. Here, we designed and synthesized two A1-D-A2-D-A1 type non-fullerene acceptors named BDTC-F and BDTC-Cl based on 1,3-bis(4-(2-ethylhexyl)-thiophen-2-yl)-5,7-bis(2-ethylhexyl)-benzo[1,2-c:4,5c']dithiophene-4,8-dione (BDD) as A2 unit. With cyclopenta[2,1-b:3,4-b']dithiophene functions as the D unit, the weak O⋯S interaction between A2 and D units is formed, which reduces the torsion angle between them and increases the planarity of the molecular backbone. Furthermore, the participation of BDD could deepen the energy levels of these two acceptors, makes it possible to decrease the energy loss of the corresponding devices. When blending with polymer donor PM6, devices based on both acceptors exhibit PCEs over 10% with low energy losses of 0.59 and 0.57 eV for BDTC-F and BDTC-Cl, respectively. Moreover, the devices based on PM6:BDTC-F and PM6:BDTC-Cl demonstrate excellent air stability. After stored in the ambient atmosphere without encapsulation for 1,200 h, devices based on these two acceptors retained over 96% of their initial PCEs. These results demonstrate that designing the A1-D-A2-D-A1 type acceptor with non-covalent intramolecular interactions is an effective strategy to construct low-cost and air-stable OSCs.
中文翻译:

基于 A 1 -DA 2 -DA 1结构的非稠环小分子受体具有低非辐射能量损失和优异的空气稳定性
近年来,有机太阳能电池(OSC) 取得了巨大进步。然而,由于光伏材料的复杂合成和器件的稳定性差,OSCs仍然存在价格质量比低的问题。与传统的大共轭梯型非富勒烯受体相比,具有非共价分子内相互作用的非稠环受体可以在保持器件性能的同时显着简化合成路线。在这里,我们基于1,3-双(4-(2-乙基己基)-噻吩-2-基)设计并合成了两种A 1 -DA 2 -DA 1型非富勒烯受体BDTC-F和BDTC-Cl) -5,7-双(2-乙基己基)-苯并[1,2-c:4,5c']dithiophene-4,8-dione (BDD) as A 2单元。以环戊二烯[2,1-b:3,4-b']二噻吩作为D单元,A 2之间的弱O⋯S相互作用形成D单元,减小了它们之间的扭转角,增加了分子骨架的平面度。此外,BDD的参与可以加深这两个受体的能级,从而减少相应器件的能量损失。当与聚合物供体 PM6 混合时,基于两种受体的器件表现出超过 10% 的 PCE,BDTC-F 和 BDTC-Cl 的能量损失分别为 0.59 和 0.57 eV。此外,基于 PM6:BDTC-F 和 PM6:BDTC-Cl 的设备表现出优异的空气稳定性。在未封装的环境中储存 1200 小时后,基于这两种受体的器件保留了其初始 PCE 的 96% 以上。这些结果表明,设计 A 1 -DA 2 -DA 1 具有非共价分子内相互作用的类型受体是构建低成本和空气稳定 OSC 的有效策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号