当前位置:
X-MOL 学术
›
J. Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Capacity for increased surface area in the hydrophobic core of β-sheet peptide bilayer nanoribbons
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2021-06-20 , DOI: 10.1002/psc.3334 Christopher W Jones 1 , Crystal G Morales 2 , Sharon L Eltiste 3 , Francine E Yanchik-Slade 1 , Naomi R Lee 3 , Bradley L Nilsson 1
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2021-06-20 , DOI: 10.1002/psc.3334 Christopher W Jones 1 , Crystal G Morales 2 , Sharon L Eltiste 3 , Francine E Yanchik-Slade 1 , Naomi R Lee 3 , Bradley L Nilsson 1
Affiliation
|
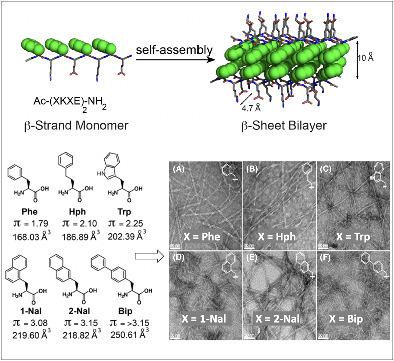
Amphipathic peptides with amino acids arranged in alternating patterns of hydrophobic and hydrophilic residues efficiently self-assemble into β-sheet bilayer nanoribbons. Hydrophobic side chain functionality is effectively buried in the interior of the putative bilayer of these nanoribbons. This study investigates consequences on self-assembly of increasing the surface area of aromatic side chain groups that reside in the hydrophobic core of nanoribbons derived from Ac-(XKXE)2-NH2 peptides (X = hydrophobic residue). A series of Ac-(XKXE)2-NH2 peptides incorporating aromatic amino acids of increasing molecular volume and steric profile (X = phenylalanine [Phe], homophenylalanine [Hph], tryptophan [Trp], 1-naphthylalanine [1-Nal], 2-naphthylalanine [2-Nal], or biphenylalanine [Bip]) were assessed to determine substitution effects on self-assembly propensity and on morphology of the resulting nanoribbon structures. Additional studies were conducted to determine the effects of incorporating amino acids of differing steric profile in the hydrophobic core (Ac-X1KFEFKFE-NH2 and Ac-(X1,5KFE)-NH2 peptides, X = Trp or Bip). Spectroscopic analysis by circular dichroism (CD) and Fourier transform infrared (FT-IR) spectroscopy indicated β-sheet formation for all variants. Self-assembly rate increased with peptide hydrophobicity; increased molecular volume of the hydrophobic side chain groups did not appear to induce kinetic penalties on self-assembly rates. Transmission electron microscopy (TEM) imaging indicated variation in fibril morphology as a function of amino acid in the X positions. This study confirms that hydrophobicity of amphipathic Ac-(XKXE)2-NH2 peptides correlates to self-assembly propensity and that the hydrophobic core of the resulting nanoribbon bilayers has a significant capacity to accommodate sterically demanding functional groups. These findings provide insight that may be used to guide the exploitation of self-assembled amphipathic peptides as functional biomaterials.
中文翻译:

增加β-折叠肽双层纳米带疏水核心表面积的能力
具有以疏水和亲水残基交替模式排列的氨基酸的两亲肽有效地自组装成β-折叠双层纳米带。疏水侧链官能团有效地埋在这些纳米带的假定双层内部。本研究调查了增加芳族侧链基团表面积对自组装的影响,这些芳族侧链基团位于源自 Ac-(XKXE) 2 -NH 2肽(X = 疏水残基)的纳米带的疏水核心中。Ac-(XKXE) 2 -NH 2系列包含增加分子体积和空间分布的芳香族氨基酸的肽(X = 苯丙氨酸 [Phe]、高苯丙氨酸 [Hph]、色氨酸 [Trp]、1-萘丙氨酸 [1-Nal]、2-萘丙氨酸 [2-Nal] 或联苯丙氨酸[Bip]) 被评估以确定取代对自组装倾向和所得纳米带结构形态的影响。进行了额外的研究以确定将不同空间分布的氨基酸掺入疏水核心(Ac-X 1 KFEFKFE-NH 2和 Ac-(X 1,5 KFE)-NH 2肽,X = Trp 或 Bip)中的效果. 圆二色性 (CD) 和傅里叶变换红外 (FT-IR) 光谱的光谱分析表明β-所有变体的表形成。自组装率随着肽疏水性的增加而增加;增加的疏水侧链基团的分子体积似乎不会引起自组装速率的动力学损失。透射电子显微镜 (TEM) 成像表明原纤维形态的变化作为 X 位氨基酸的函数。该研究证实两亲性Ac-(XKXE) 2 -NH 2肽的疏水性与自组装倾向相关,并且所得纳米带双层的疏水核心具有适应空间要求高的官能团的显着能力。这些发现提供了可用于指导开发自组装两亲性肽作为功能性生物材料的见解。
更新日期:2021-08-07
中文翻译:

增加β-折叠肽双层纳米带疏水核心表面积的能力
具有以疏水和亲水残基交替模式排列的氨基酸的两亲肽有效地自组装成β-折叠双层纳米带。疏水侧链官能团有效地埋在这些纳米带的假定双层内部。本研究调查了增加芳族侧链基团表面积对自组装的影响,这些芳族侧链基团位于源自 Ac-(XKXE) 2 -NH 2肽(X = 疏水残基)的纳米带的疏水核心中。Ac-(XKXE) 2 -NH 2系列包含增加分子体积和空间分布的芳香族氨基酸的肽(X = 苯丙氨酸 [Phe]、高苯丙氨酸 [Hph]、色氨酸 [Trp]、1-萘丙氨酸 [1-Nal]、2-萘丙氨酸 [2-Nal] 或联苯丙氨酸[Bip]) 被评估以确定取代对自组装倾向和所得纳米带结构形态的影响。进行了额外的研究以确定将不同空间分布的氨基酸掺入疏水核心(Ac-X 1 KFEFKFE-NH 2和 Ac-(X 1,5 KFE)-NH 2肽,X = Trp 或 Bip)中的效果. 圆二色性 (CD) 和傅里叶变换红外 (FT-IR) 光谱的光谱分析表明β-所有变体的表形成。自组装率随着肽疏水性的增加而增加;增加的疏水侧链基团的分子体积似乎不会引起自组装速率的动力学损失。透射电子显微镜 (TEM) 成像表明原纤维形态的变化作为 X 位氨基酸的函数。该研究证实两亲性Ac-(XKXE) 2 -NH 2肽的疏水性与自组装倾向相关,并且所得纳米带双层的疏水核心具有适应空间要求高的官能团的显着能力。这些发现提供了可用于指导开发自组装两亲性肽作为功能性生物材料的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号