当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nonsteroidal anti-inflammatory drugs based new 1,2,3-triazole derivatives: Their design, one-pot synthesis and in vitro evaluation
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2021-06-19 , DOI: 10.1002/jhet.4328 Naveen Kuntala 1 , Jyoti Mareddy 2 , Jhonsee Rani Telu 1 , Venkanna Banothu 3 , Sarbani Pal 2 , Jaya Shree Anireddy 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2021-06-19 , DOI: 10.1002/jhet.4328 Naveen Kuntala 1 , Jyoti Mareddy 2 , Jhonsee Rani Telu 1 , Venkanna Banothu 3 , Sarbani Pal 2 , Jaya Shree Anireddy 1
Affiliation
|
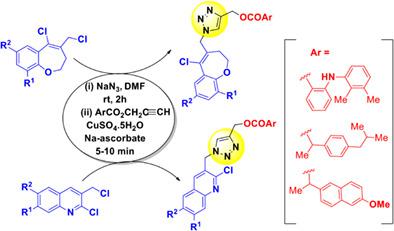
A series of novel and small molecules containing structural features of some well-known nonsteroidal anti-inflammatory drugs (NSAIDs) such as mefenamic acid or ibuprofen or naproxen and a heterocyclic moiety, for example, benzoxepine or quinoline were designed where a substituted 1,2,3-triazole was used as a linker. These molecules were initially designed as potential antibacterial agents. Synthesis of these compounds was carried out using a Cu-catalyzed azide-alkyne cycloaddition (CuAAC) as the key step to construct the central 1,2,3-triazole ring. The methodology involved regioselective and in situ azide formation from a dichloro derivative of heterocycle followed by click reaction with the appropriate alkyne containing the NSAID framework in the same pot. This one-pot sequential reaction afforded 24 target compounds in good yields. To assess their antibacterial properties, all the synthesized compounds were tested against both Gram-positive and Gram-negative species bacterial strains. One compound was found to be effective against the Gram-negative species P. aeruginosa. This compound also showed selective cytotoxicity against two cancer cell lines (COLO-205 and HOP-62) but no significant effect against noncancerous cell line (HEK293).
中文翻译:

基于非甾体抗炎药的新型 1,2,3-三唑衍生物:它们的设计、一锅合成和体外评价
设计了一系列包含一些众所周知的非甾体抗炎药 (NSAID) 结构特征的新型小分子,例如甲芬那酸或布洛芬或萘普生以及杂环部分,例如苯氧西平或喹啉,其中取代的 1,2 ,3-三唑用作接头。这些分子最初被设计为潜在的抗菌剂。这些化合物的合成是使用铜催化的叠氮化物-炔环加成 (CuAAC) 作为构建中心 1,2,3-三唑环的关键步骤进行的。该方法涉及从杂环的二氯衍生物形成区域选择性和原位叠氮化物,然后在同一锅中与含有 NSAID 框架的适当炔烃发生点击反应。这种一锅连续反应以良好的收率得到了 24 种目标化合物。为了评估它们的抗菌特性,所有合成的化合物都针对革兰氏阳性和革兰氏阴性物种细菌菌株进行了测试。发现一种化合物对革兰氏阴性菌有效铜绿假单胞菌。该化合物还显示出对两种癌细胞系(COLO-205 和 HOP-62)的选择性细胞毒性,但对非癌细胞系(HEK293)没有显着影响。
更新日期:2021-06-19
中文翻译:

基于非甾体抗炎药的新型 1,2,3-三唑衍生物:它们的设计、一锅合成和体外评价
设计了一系列包含一些众所周知的非甾体抗炎药 (NSAID) 结构特征的新型小分子,例如甲芬那酸或布洛芬或萘普生以及杂环部分,例如苯氧西平或喹啉,其中取代的 1,2 ,3-三唑用作接头。这些分子最初被设计为潜在的抗菌剂。这些化合物的合成是使用铜催化的叠氮化物-炔环加成 (CuAAC) 作为构建中心 1,2,3-三唑环的关键步骤进行的。该方法涉及从杂环的二氯衍生物形成区域选择性和原位叠氮化物,然后在同一锅中与含有 NSAID 框架的适当炔烃发生点击反应。这种一锅连续反应以良好的收率得到了 24 种目标化合物。为了评估它们的抗菌特性,所有合成的化合物都针对革兰氏阳性和革兰氏阴性物种细菌菌株进行了测试。发现一种化合物对革兰氏阴性菌有效铜绿假单胞菌。该化合物还显示出对两种癌细胞系(COLO-205 和 HOP-62)的选择性细胞毒性,但对非癌细胞系(HEK293)没有显着影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号