当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile synthesis and antimicrobial evaluations of some novel pyrazolo[3,4-b]selenolo[3,2-e]pyrazines and their related heterocycles
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2021-06-19 , DOI: 10.1002/jhet.4330 Mokhtar A. Abd ul‐Malik 1 , Adel M. Kamal El‐Dean 2 , Shaban M. Radwan 2 , Remon M. Zaki 2
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2021-06-19 , DOI: 10.1002/jhet.4330 Mokhtar A. Abd ul‐Malik 1 , Adel M. Kamal El‐Dean 2 , Shaban M. Radwan 2 , Remon M. Zaki 2
Affiliation
|
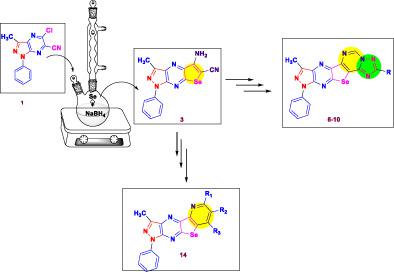
A new series of pyrazolopyrazinoselenolotriazolopyrimidines was synthesized by a facile method based on condensation of 5-amino-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]selenolo[3,2-e]pyrazine-6-carbonitrile (3) with triethyl orthoformate followed by intramolecular cyclization with hydrazine to afford 7-amino-8-imino-3-methyl-1-phenyl-1,8-dihydro-7H-pyrazolo[3″,4″:5′,6′]pyrazino[2′,3′:4,5] selenolo[3,2-d]pyrimidine (5). The latter compound was utilized as a multipurpose precursor for the construction of other new triazoles fused to the pyrazolopyrazino- selenolopyrimidine moiety. Alternatively, acetylation and chloro-acetylation of compound 3 using acetic anhydride and chloroacetyl chloride yielded the acetyl amino 11 and chloroacetamido 12 derivatives, respectively. Compound 12 underwent nucleophilic substitution upon reaction with morpholine to provide the morpholinyl acetamide 13. Furthermore, the pyrazolopyridoselenolopyrazine ring system 14 was synthesized by the reaction of the o-amino-carbonitrile 3 with malononitrile. Assignment of the chemical structures for the new compounds was confirmed depending on elemental and spectral techniques. On the other hand, most of the synthesized compounds revealed promising results against various bacterial and fungal strains.
中文翻译:

一些新型吡唑并[3,4-b]硒并[3,2-e]吡嗪及其相关杂环的简便合成和抗菌评价
基于5-氨基-3-甲基-1-苯基-1H-吡唑并[3,4- b ]硒并[3,2 - e ]吡嗪-6-缩合的简便方法合成了一系列新的吡唑并吡嗪硒并三唑并嘧啶。腈 ( 3 ) 与原甲酸三乙酯,然后与肼进行分子内环化,得到 7-amino-8-imino-3-methyl-1-phenyl-1,8-dihydro-7 H - pyrazolo[3″,4″:5' ,6']吡嗪并[2',3':4,5]硒并[3,2- d ]嘧啶( 5 )。后一种化合物被用作多用途前体,用于构建与吡唑并吡嗪基-硒并嘧啶部分稠合的其他新三唑。或者,化合物3的乙酰化和氯乙酰化使用乙酸酐和氯乙酰氯分别产生乙酰氨基11和氯乙酰氨基12衍生物。化合物12在与吗啉反应后经历亲核取代以提供吗啉基乙酰胺13。此外,通过邻氨基甲腈3与丙二腈的反应合成了吡唑并吡啶并硒代吡嗪环系14。根据元素和光谱技术确认了新化合物的化学结构归属。另一方面,大多数合成的化合物显示出针对各种细菌和真菌菌株的有希望的结果。
更新日期:2021-06-19
中文翻译:

一些新型吡唑并[3,4-b]硒并[3,2-e]吡嗪及其相关杂环的简便合成和抗菌评价
基于5-氨基-3-甲基-1-苯基-1H-吡唑并[3,4- b ]硒并[3,2 - e ]吡嗪-6-缩合的简便方法合成了一系列新的吡唑并吡嗪硒并三唑并嘧啶。腈 ( 3 ) 与原甲酸三乙酯,然后与肼进行分子内环化,得到 7-amino-8-imino-3-methyl-1-phenyl-1,8-dihydro-7 H - pyrazolo[3″,4″:5' ,6']吡嗪并[2',3':4,5]硒并[3,2- d ]嘧啶( 5 )。后一种化合物被用作多用途前体,用于构建与吡唑并吡嗪基-硒并嘧啶部分稠合的其他新三唑。或者,化合物3的乙酰化和氯乙酰化使用乙酸酐和氯乙酰氯分别产生乙酰氨基11和氯乙酰氨基12衍生物。化合物12在与吗啉反应后经历亲核取代以提供吗啉基乙酰胺13。此外,通过邻氨基甲腈3与丙二腈的反应合成了吡唑并吡啶并硒代吡嗪环系14。根据元素和光谱技术确认了新化合物的化学结构归属。另一方面,大多数合成的化合物显示出针对各种细菌和真菌菌株的有希望的结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号