Organic Materials Pub Date : 2021-06-18 , DOI: 10.1055/s-0041-1730899 Sinu C Rajappan 1 , Olav Vestrheim 1 , Mona Sharafi 1 , Jianing Li 1 , Severin T Schneebeli 1
|
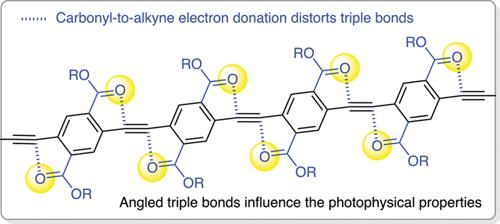
We synthesized some of the longest unimolecular oligo(p-phenylene ethynylenes) (OPEs), which are fully substituted with electron-withdrawing ester groups. An iterative convergent/divergent (a.k.a. iterative exponential growth – IEG) strategy based on Sonogashira couplings was utilized to access these sequence-defined macromolecules with up to 16 repeating units and 32 ester substituents. The carbonyl groups of the ester substituents interact with the triple bonds of the OPEs, leading to (i) unusual, angled triple bonds with increased rotational barrier, (ii) enhanced conformational disorder, and (iii) associated broadening of the UV/Vis absorption spectrum. Our results demonstrate that fully air-stable, unimolecular OPEs with ester groups can readily be accessed with IEG chemistry, providing new macromolecular backbones with unique geometrical, conformational, and photophysical properties.
中文翻译:

长达 10 nm 的单分子寡聚体(对亚苯基乙炔基)中的羰基到炔烃的给电子效应
我们合成了一些最长的单分子低聚(对亚苯基乙炔基)(OPE),它们完全被吸电子酯基取代。利用基于 Sonogashira 耦合的迭代收敛/发散(又名迭代指数增长 - IEG)策略来访问这些具有多达 16 个重复单元和 32 个酯取代基的序列定义的大分子。酯取代基的羰基与 OPE 的三键相互作用,导致 (i) 不寻常的、有角度的三键增加旋转势垒,(ii) 增强构象紊乱,以及 (iii) 相关的紫外/可见光吸收展宽光谱。我们的结果表明,完全空气稳定的带有酯基的单分子 OPE 可以通过 IEG 化学轻松获得,从而提供具有独特几何、构象和光物理性质的新大分子主链。










































 京公网安备 11010802027423号
京公网安备 11010802027423号