当前位置:
X-MOL 学术
›
J. Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and evaluation of peptide-imidazo[1,2-a]pyrazine bioconjugates as potential bivalent inhibitors of the VirB11 ATPase HP0525
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2021-06-17 , DOI: 10.1002/psc.3353 James R Sayer 1, 2 , Karin Walldén 3, 4 , Hans Koss 1, 5 , Helen Allan 1 , Tina Daviter 6, 7 , Paul J Gane 8, 9 , Gabriel Waksman 3 , Alethea B Tabor 1
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2021-06-17 , DOI: 10.1002/psc.3353 James R Sayer 1, 2 , Karin Walldén 3, 4 , Hans Koss 1, 5 , Helen Allan 1 , Tina Daviter 6, 7 , Paul J Gane 8, 9 , Gabriel Waksman 3 , Alethea B Tabor 1
Affiliation
|
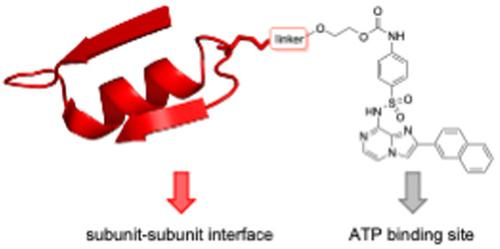
Helicobacter pylori (H. pylori) infections have been implicated in the development of gastric ulcers and various cancers: however, the success of current therapies is compromised by rising antibiotic resistance. The virulence and pathogenicity of H. pylori is mediated by the type IV secretion system (T4SS), a multiprotein macromolecular nanomachine that transfers toxic bacterial factors and plasmid DNA between bacterial cells, thus contributing to the spread of antibiotic resistance. A key component of the T4SS is the VirB11 ATPase HP0525, which is a hexameric protein assembly. We have previously reported the design and synthesis of a series of novel 8-amino imidazo[1,2-a]pyrazine derivatives as inhibitors of HP0525. In order to improve their selectivity, and potentially develop these compounds as tools for probing the assembly of the HP0525 hexamer, we have explored the design and synthesis of potential bivalent inhibitors. We used the structural details of the subunit–subunit interactions within the HP0525 hexamer to design peptide recognition moieties of the subunit interface. Different methods (cross metathesis, click chemistry, and cysteine-malemide) for bioconjugation to selected 8-amino imidazo[1,2-a]pyrazines were explored, as well as peptides spanning larger or smaller regions of the interface. The IC50 values of the resulting linker-8-amino imidazo[1,2-a]pyrazine derivatives, and the bivalent inhibitors, were related to docking studies with the HP0525 crystal structure and to molecular dynamics simulations of the peptide recognition moieties.
中文翻译:

肽-咪唑并[1,2-a]吡嗪生物偶联物作为 VirB11 ATPase HP0525 潜在二价抑制剂的设计、合成和评估
幽门螺杆菌( H. pylori ) 感染与胃溃疡和各种癌症的发展有关:然而,当前治疗的成功受到抗生素耐药性上升的影响。H. pylori的毒力和致病性由 IV 型分泌系统 (T4SS) 介导,T4SS 是一种多蛋白大分子纳米机器,可在细菌细胞之间转移有毒细菌因子和质粒 DNA,从而促进抗生素耐药性的传播。T4SS 的一个关键组成部分是 VirB11 ATPase HP0525,它是一种六聚体蛋白质组装体。我们之前报道了一系列新型8-氨基咪唑[1,2- a]吡嗪衍生物作为HP0525的抑制剂。为了提高它们的选择性,并有可能开发这些化合物作为探测 HP0525 六聚体组装的工具,我们探索了潜在二价抑制剂的设计和合成。我们使用 HP0525 六聚体中亚基-亚基相互作用的结构细节来设计亚基界面的肽识别部分。探索了不同的方法(交叉复分解、点击化学和半胱氨酸-马来酰亚胺)与选定的 8-氨基咪唑并[1,2- a ]吡嗪以及跨越较大或较小界面区域的肽进行生物共轭。所得接头-8-氨基咪唑[1,2- a ]的IC 50值]吡嗪衍生物和二价抑制剂与HP0525晶体结构的对接研究和肽识别部分的分子动力学模拟有关。
更新日期:2021-06-17
中文翻译:

肽-咪唑并[1,2-a]吡嗪生物偶联物作为 VirB11 ATPase HP0525 潜在二价抑制剂的设计、合成和评估
幽门螺杆菌( H. pylori ) 感染与胃溃疡和各种癌症的发展有关:然而,当前治疗的成功受到抗生素耐药性上升的影响。H. pylori的毒力和致病性由 IV 型分泌系统 (T4SS) 介导,T4SS 是一种多蛋白大分子纳米机器,可在细菌细胞之间转移有毒细菌因子和质粒 DNA,从而促进抗生素耐药性的传播。T4SS 的一个关键组成部分是 VirB11 ATPase HP0525,它是一种六聚体蛋白质组装体。我们之前报道了一系列新型8-氨基咪唑[1,2- a]吡嗪衍生物作为HP0525的抑制剂。为了提高它们的选择性,并有可能开发这些化合物作为探测 HP0525 六聚体组装的工具,我们探索了潜在二价抑制剂的设计和合成。我们使用 HP0525 六聚体中亚基-亚基相互作用的结构细节来设计亚基界面的肽识别部分。探索了不同的方法(交叉复分解、点击化学和半胱氨酸-马来酰亚胺)与选定的 8-氨基咪唑并[1,2- a ]吡嗪以及跨越较大或较小界面区域的肽进行生物共轭。所得接头-8-氨基咪唑[1,2- a ]的IC 50值]吡嗪衍生物和二价抑制剂与HP0525晶体结构的对接研究和肽识别部分的分子动力学模拟有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号