当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Study on Oxidative DNA Damage and Modifications by Haloquinoid Carcinogenic Intermediates and Disinfection Byproducts
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2021-06-18 , DOI: 10.1021/acs.chemrestox.1c00158 Ben-Zhan Zhu 1, 2 , Miao Tang 1, 2 , Chun-Hua Huang 1, 2 , Li Mao 1, 2 , Jie Shao 1, 2
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2021-06-18 , DOI: 10.1021/acs.chemrestox.1c00158 Ben-Zhan Zhu 1, 2 , Miao Tang 1, 2 , Chun-Hua Huang 1, 2 , Li Mao 1, 2 , Jie Shao 1, 2
Affiliation
|
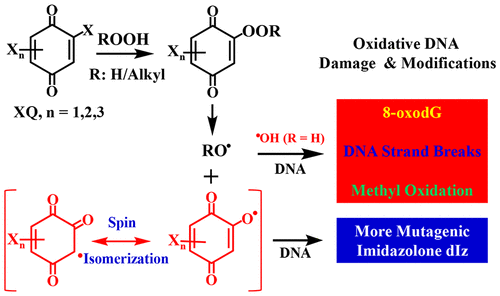
Haloquinones (XQs) are a group of carcinogenic intermediates of the haloaromatic environmental pollutants and newly identified chlorination disinfection byproducts (DBPs) in drinking water. The highly reactive hydroxyl radicals/alkoxyl radicals and quinone enoxy/ketoxy radicals were found to arise in XQs and H2O2 or organic hydroperoxides system, independent of transition-metal ions. However, it was not clear whether these haloquinoid carcinogens and hydroperoxides can cause oxidative DNA damage and modifications, and if so, what are the underlying molecular mechanisms. We found that 8-oxodeoxyguanosine (8-oxodG), DNA strand breaks, and three methyl oxidation products could arise when DNA was treated with tetrachloro-1,4-benzoquinone and H2O2 via a metal-independent and intercalation-enhanced oxidation mechanism. Similar effects were observed with other XQs, which are generally more efficient than the typical Fenton system. We further extended our studies from isolated DNA to genomic DNA in living cells. We also found that potent oxidation of DNA to the more mutagenic imidazolone dIz could be induced by XQs and organic hydroperoxides such as t-butylhydroperoxide or the physiologically relevant hydroperoxide 13S-hydroperoxy-9Z,11E-octadecadienoic acid via an unprecedented quinone-enoxy radical-mediated mechanism. These findings should provide new perspectives to explain the potential genotoxicity, mutagenesis, and carcinogenicity for the ubiquitous haloquinoid carcinogenic intermediates and DBPs.
中文翻译:

卤喹酮类致癌中间体和消毒副产物对 DNA 氧化损伤和修饰的机理研究
卤醌(XQs)是卤代环环境污染物和饮用水中新发现的氯化消毒副产物(DBPs)的一组致癌中间体。发现在 XQ 和 H 2 O 2或有机氢过氧化物体系中产生高反应性羟基自由基/烷氧基自由基和醌烯氧基/酮氧基自由基,与过渡金属离子无关。然而,尚不清楚这些卤喹啉类致癌物和氢过氧化物是否会导致氧化性 DNA 损伤和修饰,如果会,潜在的分子机制是什么。我们发现当 DNA 用四氯-1,4-苯醌和 H 2 O 2处理时,会产生 8-氧代脱氧鸟苷 (8-oxodG)、DNA 链断裂和三种甲基氧化产物通过独立于金属和嵌入增强的氧化机制。其他 XQ 也观察到了类似的效果,它们通常比典型的 Fenton 系统更有效。我们进一步将研究从分离的 DNA 扩展到活细胞中的基因组 DNA。我们还发现 XQ 和有机氢过氧化物(如叔丁基氢过氧化物或生理相关氢过氧化物 13S-氢过氧-9Z, 11E-十八碳二烯酸)可通过前所未有的醌-烯氧基自由基诱导 DNA 有效氧化为更具致突变性的咪唑酮 dIz。介导的机制。这些发现应该为解释普遍存在的卤喹啉致癌中间体和 DBP 的潜在遗传毒性、诱变和致癌性提供新的视角。
更新日期:2021-07-19
中文翻译:

卤喹酮类致癌中间体和消毒副产物对 DNA 氧化损伤和修饰的机理研究
卤醌(XQs)是卤代环环境污染物和饮用水中新发现的氯化消毒副产物(DBPs)的一组致癌中间体。发现在 XQ 和 H 2 O 2或有机氢过氧化物体系中产生高反应性羟基自由基/烷氧基自由基和醌烯氧基/酮氧基自由基,与过渡金属离子无关。然而,尚不清楚这些卤喹啉类致癌物和氢过氧化物是否会导致氧化性 DNA 损伤和修饰,如果会,潜在的分子机制是什么。我们发现当 DNA 用四氯-1,4-苯醌和 H 2 O 2处理时,会产生 8-氧代脱氧鸟苷 (8-oxodG)、DNA 链断裂和三种甲基氧化产物通过独立于金属和嵌入增强的氧化机制。其他 XQ 也观察到了类似的效果,它们通常比典型的 Fenton 系统更有效。我们进一步将研究从分离的 DNA 扩展到活细胞中的基因组 DNA。我们还发现 XQ 和有机氢过氧化物(如叔丁基氢过氧化物或生理相关氢过氧化物 13S-氢过氧-9Z, 11E-十八碳二烯酸)可通过前所未有的醌-烯氧基自由基诱导 DNA 有效氧化为更具致突变性的咪唑酮 dIz。介导的机制。这些发现应该为解释普遍存在的卤喹啉致癌中间体和 DBP 的潜在遗传毒性、诱变和致癌性提供新的视角。











































 京公网安备 11010802027423号
京公网安备 11010802027423号