当前位置:
X-MOL 学术
›
Chem. Rec.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Glycosyl Sulfonates Beyond Triflates
The Chemical Record ( IF 7.0 ) Pub Date : 2021-06-17 , DOI: 10.1002/tcr.202100141 Clay S Bennett 1
The Chemical Record ( IF 7.0 ) Pub Date : 2021-06-17 , DOI: 10.1002/tcr.202100141 Clay S Bennett 1
Affiliation
|
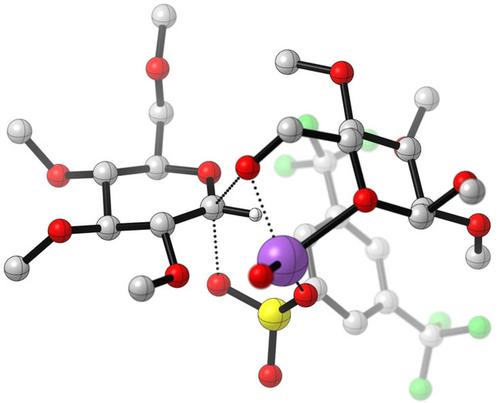
While glycosyl triflates are frequently invoked as intermediates in many chemical glycosylation reactions, the chemistry of other glycosyl sulfonates remains comparatively underexplored. Given the reactivity of sulfonates can span several orders of magnitude, this represents an untapped resource for the development of stereoselective glycosylation reactions. This personal account describes our laboratories efforts to take advantage of this reactivity to develop β-specific glycosylation reactions. Initial investigations led to the development of 2-deoxy-sugar tosylates as highly selective donors for β-glycoside synthesis, an approach which has been used to great success by our group and others for the construction of deoxy-sugar oligosaccharides and natural products. Subsequent studies demonstrate that “matching” the reactivity of the sulfonate to that of the sugar donor leads to highly selective SN2-glycosylations with a range of substrates.
中文翻译:

三氟甲磺酸盐以外的糖基磺酸盐
虽然糖基三氟甲磺酸酯经常在许多化学糖基化反应中用作中间体,但其他糖基磺酸盐的化学性质仍然相对未得到充分研究。鉴于磺酸盐的反应活性可以跨越几个数量级,这代表了立体选择性糖基化反应开发的未开发资源。这个个人描述描述了我们的实验室利用这种反应性来开发 β 特异性糖基化反应的努力。初步研究开发了 2-脱氧糖甲苯磺酸盐作为 β-糖苷合成的高选择性供体,该方法已被我们小组和其他人用于构建脱氧糖寡糖和天然产物,并取得了巨大成功。随后的研究表明,磺酸盐的反应性与糖供体的反应性“匹配”会导致与一系列底物的高度选择性 S N 2-糖基化。
更新日期:2021-06-17
中文翻译:

三氟甲磺酸盐以外的糖基磺酸盐
虽然糖基三氟甲磺酸酯经常在许多化学糖基化反应中用作中间体,但其他糖基磺酸盐的化学性质仍然相对未得到充分研究。鉴于磺酸盐的反应活性可以跨越几个数量级,这代表了立体选择性糖基化反应开发的未开发资源。这个个人描述描述了我们的实验室利用这种反应性来开发 β 特异性糖基化反应的努力。初步研究开发了 2-脱氧糖甲苯磺酸盐作为 β-糖苷合成的高选择性供体,该方法已被我们小组和其他人用于构建脱氧糖寡糖和天然产物,并取得了巨大成功。随后的研究表明,磺酸盐的反应性与糖供体的反应性“匹配”会导致与一系列底物的高度选择性 S N 2-糖基化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号