当前位置:
X-MOL 学术
›
Helv. Chimica Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Total Synthesis of (+)-Nordasycarpidone, (+)-Dasycarpidone, and (+)-Uleine
Helvetica Chimica Acta ( IF 1.5 ) Pub Date : 2021-06-17 , DOI: 10.1002/hlca.202100088 Bastien Delayre 1 , Cédric Fung 2 , Qian Wang 2 , Jieping Zhu 3
Helvetica Chimica Acta ( IF 1.5 ) Pub Date : 2021-06-17 , DOI: 10.1002/hlca.202100088 Bastien Delayre 1 , Cédric Fung 2 , Qian Wang 2 , Jieping Zhu 3
Affiliation
|
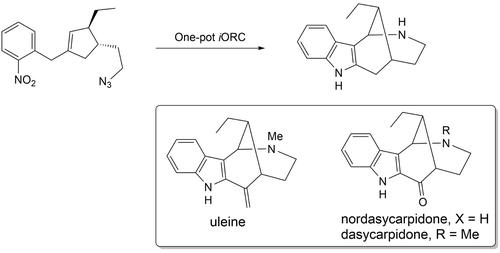
The structure of uleine type alkaloids is characterized by the presence of a bridged tetracyclic hexahydro-1H-1,5-methanoazocino[4,3-b]indole ring system 1. Various strategies have been developed to access this polycyclic structural motif. We report herein a one-step conversion of appropriately functionalized 1,3,4-trisubstituted cyclopent-1-ene to 1 by way of an integrated oxidation/reduction/cyclization (iORC) process. This domino sequence, initiated by oxidative cleavage of cyclopentene ring, generated subsequently a cyclohexenone, an indole and a 1,3-bridged piperidine ring through formation of one C−C and two C−N bonds. Compound 1 is subsequently converted to nordasycarpidone, dasycarpidone and uleine. The chirality of the molecule was introduced by enzymatic desymmetrization of commercially available meso cis-3,5-diacetoxy-1-cyclopentene.
中文翻译:

(+)-去甲苷、(+)-Dasycarpidone和(+)-Uleine的对映选择性全合成
uleine 型生物碱的结构特征在于存在桥连的四环六氢-1 H -1,5-methanoazocino[4,3- b ]indole 环系统1。已经开发了各种策略来访问这种多环结构基序。我们在此报告适当官能化的1,3,4-三取代的环戊-1-烯的一步法转化成1通过一个集成的氧化/还原/环化(的方式我ORC)过程。该多米诺序列由环戊烯环的氧化裂解引发,随后通过形成一个 C-C 和两个 CN 键生成环己烯酮、吲哚和 1,3-桥接哌啶环。化合物1随后转化为nordasycarpidone、dasycarpidone和uleine。分子的手性通过市售的内消旋-3,5-二乙酰氧基-1-环戊烯的酶促去对称化引入。
更新日期:2021-08-17
中文翻译:

(+)-去甲苷、(+)-Dasycarpidone和(+)-Uleine的对映选择性全合成
uleine 型生物碱的结构特征在于存在桥连的四环六氢-1 H -1,5-methanoazocino[4,3- b ]indole 环系统1。已经开发了各种策略来访问这种多环结构基序。我们在此报告适当官能化的1,3,4-三取代的环戊-1-烯的一步法转化成1通过一个集成的氧化/还原/环化(的方式我ORC)过程。该多米诺序列由环戊烯环的氧化裂解引发,随后通过形成一个 C-C 和两个 CN 键生成环己烯酮、吲哚和 1,3-桥接哌啶环。化合物1随后转化为nordasycarpidone、dasycarpidone和uleine。分子的手性通过市售的内消旋-3,5-二乙酰氧基-1-环戊烯的酶促去对称化引入。











































 京公网安备 11010802027423号
京公网安备 11010802027423号