Structure ( IF 4.4 ) Pub Date : 2021-06-16 , DOI: 10.1016/j.str.2021.05.015 Cristian A Escobar 1 , Badreddine Douzi 2 , Geneviève Ball 2 , Brice Barbat 2 , Sebastien Alphonse 2 , Loïc Quinton 3 , Romé Voulhoux 2 , Katrina T Forest 1
|
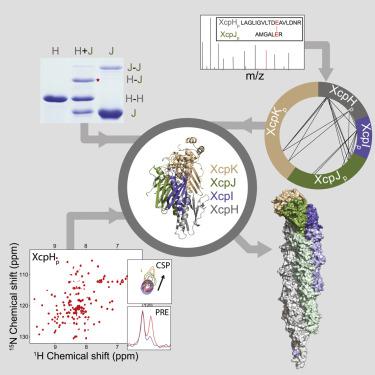
The type IV filament superfamily comprises widespread membrane-associated polymers in prokaryotes. The type II secretion system (T2SS), a virulence pathway in many pathogens, belongs to this superfamily. A knowledge gap in understanding of the T2SS is the molecular role of a small “pseudopilin” protein. Using multiple biophysical techniques, we have deciphered how this missing component of the Xcp T2SS architecture is structurally integrated, and thereby unlocked its function. We demonstrate that low-abundance XcpH is the adapter that bridges a trimeric initiating tip complex, XcpIJK, with a periplasmic filament of XcpG subunits. Each pseudopilin protein caps an XcpG protofilament in an overall pseudopilus compatible with dimensions of the periplasm and the outer membrane-spanning secretin through which substrates pass. Unexpectedly, to fulfill its adapter function, the XcpH N-terminal helix must be unwound, a property shared with XcpG subunits. We provide an experimentally validated three-dimensional structural model of a complete type IV filament.
中文翻译:

结构相互作用定义了 II 型分泌系统假纤毛蛋白的装配适配器功能
IV 型细丝超家族包括原核生物中广泛存在的膜相关聚合物。II型分泌系统(T2SS)是许多病原体的毒力途径,属于这个超家族。对 T2SS 理解的一个知识空白是一种小的“pseudopilin”蛋白的分子作用。使用多种生物物理技术,我们已经破译了 Xcp T2SS 架构中这个缺失的组件是如何在结构上集成的,从而解锁了它的功能。我们证明低丰度 XcpH 是连接三聚体起始尖端复合物 XcpIJK 与 XcpG 亚基周质细丝的适配器。每个假菌毛蛋白在整个假菌毛中覆盖 XcpG 原丝,与周质的尺寸和底物通过的外跨膜促胰液素相容。不料,为了实现其适配器功能,必须解开 XcpH N 端螺旋,这是与 XcpG 亚基共享的属性。我们提供了一个经过实验验证的完整 IV 型灯丝的三维结构模型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号