Journal of Molecular Graphics and Modelling ( IF 2.7 ) Pub Date : 2021-06-16 , DOI: 10.1016/j.jmgm.2021.107966 Payam Kalhor 1 , Ommolbanin Yarivand 2 , Kumars Seifpanahi-Shabani 3
|
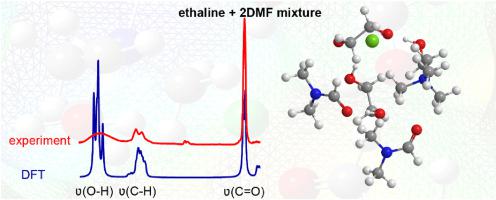
Deep-eutectic solvents (DESs) gained attention of researchers as green solvents. Making binary mixtures of DESs with appropriate cosolvents is a strategy to obtain more favorable mixtures. Here, structural features and hydrogen bonding (H-bonding) properties of binary mixtures containing ethaline (ETH) DES, (choline chloride (ChCl):2 ethylene glycol (EG)) with N,N-dimethylformamide (DMF) are reported. Such investigations are carried out by density functional theory (DFT) calculations. The results show that in ETH-DMF mixtures, DMF molecules can hardly overcome the strong Columbic interaction and doubly ionic H-bonds between the ions Ch+ and Cl− or the ionic H-bonds between Ch+ and EG. Upon EG addition to ChCl to obtain ETH or DMF addition to ETH, the Cl− … Ch+ connectivity decreases, implying charge delocalization from Cl− to other components rather than Ch+. This is supported by the blue shift of Ch+ hydroxyl observed in the calculated infrared spectra.
中文翻译:

二甲基甲酰胺在乙醇中溶解的量子化学计算
深共熔溶剂 (DESs) 作为绿色溶剂受到研究人员的关注。用适当的助溶剂制备 DES 的二元混合物是一种获得更有利混合物的策略。在这里,报告了包含乙胺 (ETH) DES、(氯化胆碱 (ChCl):2 乙二醇 (EG))与N , N-二甲基甲酰胺 (DMF)的二元混合物的结构特征和氢键(H 键)特性。此类调查是通过密度泛函理论 (DFT) 计算进行的。结果表明,在 ETH-DMF 混合物中,DMF 分子很难克服强 Columbic 相互作用和离子 Ch +和 Cl -之间的双离子 H 键或 Ch +之间的离子 H 键。和EG。在将 EG 添加到 ChCl 以获得 ETH 或 DMF 添加到 ETH 后,Cl - ... Ch +连接性降低,这意味着电荷从 Cl -到其他组分而不是 Ch + 的离域。在计算的红外光谱中观察到的 Ch +羟基的蓝移支持了这一点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号