当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental Study of Semiclathrate Hydrates Formation TBAOH, TBAF, and TBAC in the Presence of SDS and Tween Surfactants as a Cold Thermal Energy Storage System for Air Conditioning Applications
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-06-16 , DOI: 10.1021/acs.jced.1c00365 Ahmad Khafaei 1 , Arash Kamran-Pirzaman 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-06-16 , DOI: 10.1021/acs.jced.1c00365 Ahmad Khafaei 1 , Arash Kamran-Pirzaman 1
Affiliation
|
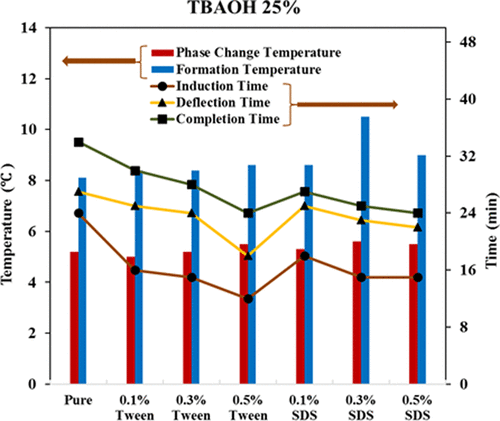
Over the past decades, the increasing use of air conditioning systems has been one of the main drivers for reducing the peak of electricity consumption. PCMs (phase change materials) for cold thermal energy storage (CTES) allow us to achieve a heat capacity for future use. Hence, CTES has been suggested to reduce power demand problems. In this study, to obtain the phase change temperature and formation temperature of TBAF, TBAC, and TBAOH semiclathrate salts as CTES for air conditioning applications, the experiments were performed from 10 to 50 wt %. Also, the effect of two additives, Tween 80 and SDS, at three weight percentages (0.1, 0.3, and 0.5 wt %) on formation temperature and phase change temperature was investigated. Furthermore, the effects of SDS and Tween mixtures using Design Expert software and Analysis of Variance (ANOVA) were analyzed and optimized. The results showed that, in general, TBAF hydrate solution had a higher phase change temperature and formation temperature than TBAC and TBAOH salt semiclathrate. The highest phase change temperature for all salts TBAF, TBAC, and TBOAH occurred at approximately 35 wt %, and their temperatures were 24.6 °C, 14.1 °C, and 12.8 °C, respectively. Using SDS and Tween additives, the phase change temperature and hydrate deflection time of TBAC, TBAF, and TBAOH were 21.73 °C, 26.08 °C, and 28.84 °C and 32.31 min, 25.92 min, and 20.20 min, respectively. With the Design Expert software, a model for the phase change temperature and deflection time of each hydrate was obtained. In the optimization section, a condition of the optimal hydrate formation was proposed.
中文翻译:

在 SDS 和吐温表面活性剂存在下,半包合物水合物形成 TBAOH、TBAF 和 TBAC 的实验研究作为空调应用的冷热储能系统
在过去的几十年里,越来越多地使用空调系统一直是减少用电高峰的主要驱动力之一。用于冷热能储存 (CTES) 的 PCM(相变材料)使我们能够实现未来使用的热容量。因此,已建议使用 CTES 来减少电力需求问题。在这项研究中,为了获得 TBAF、TBAC 和 TBAOH 半包合物盐作为用于空调应用的 CTES 的相变温度和形成温度,实验进行了 10 至 50 重量%。此外,研究了三种重量百分比(0.1、0.3 和 0.5 重量%)的两种添加剂吐温 80 和 SDS 对地层温度和相变温度的影响。此外,使用 Design Expert 软件和方差分析 (ANOVA) 对 SDS 和吐温混合物的影响进行了分析和优化。结果表明,总的来说,TBAF水合物溶液比TBAC和TBAOH盐半包合物具有更高的相变温度和地层温度。所有盐 TBAF、TBAC 和 TBOAH 的最高相变温度发生在大约 35 wt% 时,它们的温度分别为 24.6 °C、14.1 °C 和 12.8 °C。使用 SDS 和 Tween 添加剂,TBAC、TBAF 和 TBAOH 的相变温度和水合物偏转时间分别为 21.73 °C、26.08 °C 和 28.84 °C,以及 32.31 min、25.92 min 和 20.20 min。使用Design Expert软件,获得了每种水合物的相变温度和偏转时间模型。在优化部分,
更新日期:2021-07-08
中文翻译:

在 SDS 和吐温表面活性剂存在下,半包合物水合物形成 TBAOH、TBAF 和 TBAC 的实验研究作为空调应用的冷热储能系统
在过去的几十年里,越来越多地使用空调系统一直是减少用电高峰的主要驱动力之一。用于冷热能储存 (CTES) 的 PCM(相变材料)使我们能够实现未来使用的热容量。因此,已建议使用 CTES 来减少电力需求问题。在这项研究中,为了获得 TBAF、TBAC 和 TBAOH 半包合物盐作为用于空调应用的 CTES 的相变温度和形成温度,实验进行了 10 至 50 重量%。此外,研究了三种重量百分比(0.1、0.3 和 0.5 重量%)的两种添加剂吐温 80 和 SDS 对地层温度和相变温度的影响。此外,使用 Design Expert 软件和方差分析 (ANOVA) 对 SDS 和吐温混合物的影响进行了分析和优化。结果表明,总的来说,TBAF水合物溶液比TBAC和TBAOH盐半包合物具有更高的相变温度和地层温度。所有盐 TBAF、TBAC 和 TBOAH 的最高相变温度发生在大约 35 wt% 时,它们的温度分别为 24.6 °C、14.1 °C 和 12.8 °C。使用 SDS 和 Tween 添加剂,TBAC、TBAF 和 TBAOH 的相变温度和水合物偏转时间分别为 21.73 °C、26.08 °C 和 28.84 °C,以及 32.31 min、25.92 min 和 20.20 min。使用Design Expert软件,获得了每种水合物的相变温度和偏转时间模型。在优化部分,











































 京公网安备 11010802027423号
京公网安备 11010802027423号