Journal of the Energy Institute ( IF 5.6 ) Pub Date : 2021-06-15 , DOI: 10.1016/j.joei.2021.06.002 Yiran Wang , Kai Sun , Shu Zhang , Leilei Xu , Guangzhi Hu , Xun Hu
|
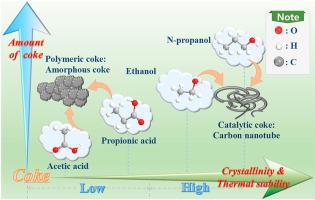
Alcohols and carboxylic acids have been used intensively as feedstock for production of hydrogen via steam reforming. They have different functionalities and might exhibit the distinct coking behaviors. In this study, ethanol, n-propanol, acetic acid and propionic acid were chosen as the representatives of alcohols/carboxylic acids and their reaction characteristics in steam reforming over Ni/SBA-15 catalyst were comparatively investigated with the focus on understanding the difference of the properties of the coke formed. The results indicated that although the steam reforming of the alcohols generated more coke than that of the carboxylic acids, the catalyst showed the higher catalytic stabilities, due to different properties of the coke formed. The steam reforming of the alcohols produced the coke of higher aromaticity with a higher content of carbon, more large defective aromatic rings, higher crystallinity, higher thermal stability and resistivity towards the oxidation than that from steam reforming of the carboxylic acids. The coke in the carbon nanotube form in steam reforming of the alcohols and the carboxylic acids also differed in terms of wall thickness and length of the carbon nanotubes, as the reaction intermediates were different. The encapsulating coke was more abundant in steam reforming of carboxylic acids, as carboxylic acids are prone to form the amorphous thermal coke via cracking, which led to rapid deactivation of the catalyst. The types or properties of the coke, not only the amount, play important roles in determining stability of the catalyst.
中文翻译:

醇和羧酸的蒸汽重整:羧基和醇羟基对焦炭性能的重要性
醇和羧酸已被广泛用作通过蒸汽重整生产氢气的原料。它们具有不同的功能并且可能表现出不同的焦化行为。本研究选择乙醇、正丙醇、乙酸和丙酸作为醇/羧酸的代表,对比研究了它们在 Ni/SBA-15 催化剂上水蒸汽重整的反应特性,重点是了解醇/羧酸的差异。形成的焦炭的性质。结果表明,虽然醇的蒸汽重整产生的焦炭多于羧酸,但由于形成的焦炭的性质不同,催化剂表现出更高的催化稳定性。与羧酸蒸汽重整相比,醇的蒸汽重整产生的焦炭具有更高的芳香性,具有更高的碳含量、更大的缺陷芳环、更高的结晶度、更高的热稳定性和抗氧化性。由于反应中间体不同,醇和羧酸蒸汽重整形成的碳纳米管中的焦炭在碳纳米管的壁厚和长度方面也不同。在羧酸的蒸汽重整中,包封焦更丰富,因为羧酸易于通过裂化形成无定形热焦,这导致催化剂快速失活。焦炭的类型或性质,不仅是数量,在决定催化剂的稳定性方面起着重要作用。与羧酸的蒸汽重整相比,有更大的缺陷芳环、更高的结晶度、更高的热稳定性和抗氧化性。由于反应中间体不同,醇和羧酸蒸汽重整形成的碳纳米管中的焦炭在碳纳米管的壁厚和长度方面也不同。在羧酸的蒸汽重整中,包封焦更丰富,因为羧酸易于通过裂化形成无定形热焦,这导致催化剂快速失活。焦炭的类型或性质,不仅是数量,在决定催化剂的稳定性方面起着重要作用。与羧酸的蒸汽重整相比,有更大的缺陷芳环、更高的结晶度、更高的热稳定性和抗氧化性。由于反应中间体不同,醇和羧酸蒸汽重整形成的碳纳米管中的焦炭在碳纳米管的壁厚和长度方面也不同。在羧酸的蒸汽重整中,包封焦更丰富,因为羧酸易于通过裂化形成无定形热焦,这导致催化剂快速失活。焦炭的类型或性质,不仅是数量,在决定催化剂的稳定性方面起着重要作用。与羧酸的蒸汽重整相比,具有更高的热稳定性和抗氧化性。由于反应中间体不同,醇和羧酸蒸汽重整形成的碳纳米管中的焦炭在碳纳米管的壁厚和长度方面也不同。在羧酸的蒸汽重整中,包封焦更丰富,因为羧酸易于通过裂化形成无定形热焦,这导致催化剂快速失活。焦炭的类型或性质,不仅是数量,在决定催化剂的稳定性方面起着重要作用。与羧酸的蒸汽重整相比,具有更高的热稳定性和抗氧化性。由于反应中间体不同,醇和羧酸蒸汽重整形成的碳纳米管中的焦炭在碳纳米管的壁厚和长度方面也不同。在羧酸的蒸汽重整中,包封焦更丰富,因为羧酸易于通过裂化形成无定形热焦,这导致催化剂快速失活。焦炭的类型或性质,不仅是数量,在决定催化剂的稳定性方面起着重要作用。由于反应中间体不同,醇和羧酸蒸汽重整形成的碳纳米管中的焦炭在碳纳米管的壁厚和长度方面也不同。在羧酸的蒸汽重整中,包封焦更丰富,因为羧酸易于通过裂化形成无定形热焦,这导致催化剂快速失活。焦炭的类型或性质,不仅是数量,在决定催化剂的稳定性方面起着重要作用。由于反应中间体不同,醇和羧酸蒸汽重整形成的碳纳米管中的焦炭在碳纳米管的壁厚和长度方面也不同。在羧酸的蒸汽重整中,包封焦更丰富,因为羧酸易于通过裂化形成无定形热焦,这导致催化剂快速失活。焦炭的类型或性质,不仅是数量,在决定催化剂的稳定性方面起着重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号