Journal of Autoimmunity ( IF 7.9 ) Pub Date : 2021-06-15 , DOI: 10.1016/j.jaut.2021.102681 Jimin Hwang 1 , Se Bee Lee 2 , Seung Won Lee 3 , Min Ho Lee 4 , Ai Koyanagi 5 , Louis Jacob 6 , Kalthoum Tizaoui 7 , Dong Keon Yon 8 , Jae Il Shin 9 , Lee Smith 10
|
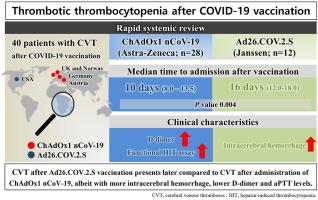
Cerebral venous thrombosis (CVT) events have been reported after vaccination with adenoviral COVID-19 vector vaccines. This study aimed to compare the clinical presentations and courses of vaccine-induced thrombotic thrombocytopenia (VITT) between the two adenoviral vector vaccines, Ad26.COV.2.S (Janssen/Johnson & Johnson) and ChAdOx1 nCoV-19 (Astra-Zeneca). We found that CVT after Ad26.COV.2.S vaccination presents later with similar symptoms compared to CVT after administration of ChAdOx1 nCoV-19, albeit with more thrombosis and intracerebral hemorrhage, lower D-dimer and aPTT levels but similar mortality. These findings could help guide clinical assessment and management of CVT after COVID-19 vaccination.
中文翻译:

ChAdOx1 nCoV-19 和 Ad26.COV.2.S 疫苗之间疫苗诱导血栓事件的比较
在接种腺病毒 COVID-19 载体疫苗后,已有脑静脉血栓形成 (CVT) 事件的报道。本研究旨在比较两种腺病毒载体疫苗 Ad26.COV.2.S(杨森/强生)和 ChAdOx1 nCoV-19(阿斯利康)之间疫苗诱导的血栓性血小板减少症 (VITT) 的临床表现和病程. 我们发现,与接种 ChAdOx1 nCoV-19 后的 CVT 相比,Ad26.COV.2.S 疫苗接种后的 CVT 出现的症状更晚,尽管血栓形成和脑出血更多,D-二聚体和 aPTT 水平更低,但死亡率相似。这些发现可能有助于指导 COVID-19 疫苗接种后 CVT 的临床评估和管理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号