当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Peracetic Acid–Ruthenium(III) Oxidation Process for the Degradation of Micropollutants in Water
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-06-15 , DOI: 10.1021/acs.est.0c06676 Ruobai Li 1 , Kyriakos Manoli 1 , Juhee Kim 2 , Mingbao Feng 1 , Ching-Hua Huang 2 , Virender K Sharma 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-06-15 , DOI: 10.1021/acs.est.0c06676 Ruobai Li 1 , Kyriakos Manoli 1 , Juhee Kim 2 , Mingbao Feng 1 , Ching-Hua Huang 2 , Virender K Sharma 1
Affiliation
|
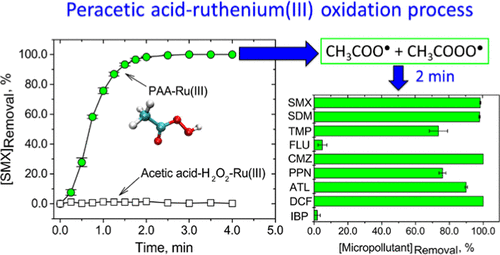
This paper presents an advanced oxidation process (AOP) of peracetic acid (PAA) and ruthenium(III) (Ru(III)) to oxidize micropollutants in water. Studies of PAA–Ru(III) oxidation of sulfamethoxazole (SMX), a sulfonamide antibiotic, in 0.5–20.0 mM phosphate solution at different pH values (5.0–9.0) showed an optimum pH of 7.0 with a complete transformation of SMX in 2.0 min. At pH 7.0, other metal ions (i.e., Fe(II), Fe(III), Mn(II), Mn(III), Co(II), Cu(II), and Ni(II)) in 10 mM phosphate could activate PAA to oxidize SMX only up to 20%. The PAA–Ru(III) oxidation process was also unaffected by the presence of chloride and carbonate ions in solution. Electron paramagnetic resonance (EPR) measurements and quenching experiments showed the dominant involvement of the acetyl(per)oxyl radicals (i.e., CH3C(O)O• and CH3C(O)OO•) for degrading SMX in the PAA–Ru(III) oxidation process. The transformation pathways of SMX by PAA–Ru(III) were proposed based on the identified intermediates. Tests with other pharmaceuticals demonstrated that the PAA–Ru(III) oxidation system could remove efficiently a wide range of pharmaceuticals (9 compounds) in the presence of phosphate ions in 2.0 min at neutral pH. The knowledge gained herein on the effective role of Ru(III) to activate PAA to oxidize micropollutants may aid in developing Ru(III)-containing catalysts for PAA-based AOPs.
中文翻译:

用于降解水中微污染物的过氧乙酸-钌 (III) 氧化工艺
本文介绍了过氧乙酸 (PAA) 和钌 (III) (Ru(III)) 的高级氧化工艺 (AOP),以氧化水中的微污染物。磺胺类抗生素磺胺甲恶唑 (SMX) 在 0.5-20.0 mM 磷酸盐溶液中在不同 pH 值 (5.0-9.0) 中 PAA-Ru(III) 氧化的研究表明,最佳 pH 值为 7.0,SMX 在 2.0 分钟内完全转化. 在 pH 7.0 时,10 mM 磷酸盐中的其他金属离子(即 Fe(II)、Fe(III)、Mn(II)、Mn(III)、Co(II)、Cu(II) 和 Ni(II)最多只能激活 PAA 氧化 SMX 20%。PAA-Ru(III) 氧化过程也不受溶液中氯离子和碳酸根离子的影响。电子顺磁共振 (EPR) 测量和猝灭实验表明乙酰(过)氧基自由基(即 CH 3 C(O)O•和 CH 3 C(O)OO • ) 用于在 PAA-Ru(III) 氧化过程中降解 SMX。基于鉴定的中间体提出了PAA-Ru(III)转化SMX的途径。对其他药物的测试表明,PAA-Ru(III) 氧化系统可以在中性 pH 值下,在磷酸根离子存在的情况下,在 2.0 分钟内有效去除多种药物(9 种化合物)。在此获得的关于 Ru(III) 激活 PAA 以氧化微污染物的有效作用的知识可能有助于开发用于基于 PAA 的 AOP 的含 Ru(III) 催化剂。
更新日期:2021-07-06
中文翻译:

用于降解水中微污染物的过氧乙酸-钌 (III) 氧化工艺
本文介绍了过氧乙酸 (PAA) 和钌 (III) (Ru(III)) 的高级氧化工艺 (AOP),以氧化水中的微污染物。磺胺类抗生素磺胺甲恶唑 (SMX) 在 0.5-20.0 mM 磷酸盐溶液中在不同 pH 值 (5.0-9.0) 中 PAA-Ru(III) 氧化的研究表明,最佳 pH 值为 7.0,SMX 在 2.0 分钟内完全转化. 在 pH 7.0 时,10 mM 磷酸盐中的其他金属离子(即 Fe(II)、Fe(III)、Mn(II)、Mn(III)、Co(II)、Cu(II) 和 Ni(II)最多只能激活 PAA 氧化 SMX 20%。PAA-Ru(III) 氧化过程也不受溶液中氯离子和碳酸根离子的影响。电子顺磁共振 (EPR) 测量和猝灭实验表明乙酰(过)氧基自由基(即 CH 3 C(O)O•和 CH 3 C(O)OO • ) 用于在 PAA-Ru(III) 氧化过程中降解 SMX。基于鉴定的中间体提出了PAA-Ru(III)转化SMX的途径。对其他药物的测试表明,PAA-Ru(III) 氧化系统可以在中性 pH 值下,在磷酸根离子存在的情况下,在 2.0 分钟内有效去除多种药物(9 种化合物)。在此获得的关于 Ru(III) 激活 PAA 以氧化微污染物的有效作用的知识可能有助于开发用于基于 PAA 的 AOP 的含 Ru(III) 催化剂。










































 京公网安备 11010802027423号
京公网安备 11010802027423号