当前位置:
X-MOL 学术
›
J. Neurochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ALS-linked mutations impair UBQLN2 stress-induced biomolecular condensate assembly in cells
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2021-06-15 , DOI: 10.1111/jnc.15453 Julia F Riley 1, 2, 3, 4 , Peter J Fioramonti 1 , Amber K Rusnock 1 , Heidi Hehnly 1 , Carlos A Castañeda 1, 2, 3, 5
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2021-06-15 , DOI: 10.1111/jnc.15453 Julia F Riley 1, 2, 3, 4 , Peter J Fioramonti 1 , Amber K Rusnock 1 , Heidi Hehnly 1 , Carlos A Castañeda 1, 2, 3, 5
Affiliation
|
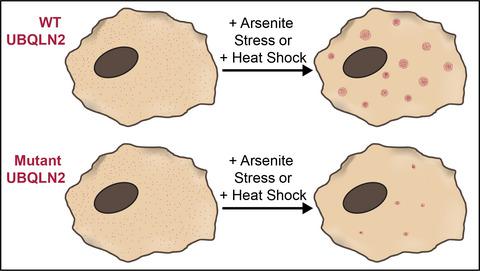
Mutations in ubiquilin-2 (UBQLN2), a ubiquitin-binding shuttle protein involved in several protein quality control processes, can lead to amyotrophic lateral sclerosis (ALS). We previously found that wild-type UBQLN2 forms dynamic, membraneless biomolecular condensates upon cellular stress, and undergoes liquid–liquid phase separation in vitro. However, the impact of ALS-linked mutations on UBQLN2 condensate formation in cells remains unknown. Here, we overexpress mCherry-fused UBQLN2 with five patient-derived ALS-linked mutations and employ live-cell imaging and photokinetic analysis to investigate how each of these mutations impact stress-induced UBQLN2 condensate assembly and condensate material properties. Unlike endogenous UBQLN2, exogenously introduced UBQLN2 forms condensates distinct from stress granules. Both wild-type and mutant UBQLN2 condensates are generally cytoplasmic and liquid-like. However, mutant UBQLN2 forms fewer stress-induced UBQLN2 condensates than wild-type UBQLN2. Exogenously expressed P506T UBQLN2 forms the lowest number of stress-induced condensates of all UBQLN2 mutants, and these condensates are significantly smaller than those of wild-type UBQLN2. Fluorescence recovery after photobleaching (FRAP) analysis of UBQLN2 condensates revealed higher immobile fractions for UBQLN2 mutants, especially P506T. P497S and P497H mutations differentially impact condensate properties, demonstrating that the effects of ALS-linked mutations are both position- and amino acid-dependent. Collectively, our data show that disease mutations hinder assembly and alter viscoelastic properties of stress-induced UBQLN2 condensates, potentially leading to aggregates commonly observed in ALS.
中文翻译:

ALS相关突变损害UBQLN2应激诱导的细胞中生物分子凝聚物组装
ubiquilin-2 (UBQLN2) 是一种参与多种蛋白质质量控制过程的泛素结合穿梭蛋白,其突变可导致肌萎缩侧索硬化症 (ALS)。我们之前发现野生型 UBQLN2 在细胞应激下形成动态的、无膜的生物分子凝聚物,并在体外进行液-液相分离。然而,ALS 相关突变对细胞中 UBQLN2 凝聚物形成的影响仍然未知。在这里,我们用五个源自患者的 ALS 相关突变过表达 mCherry 融合的 UBQLN2,并采用活细胞成像和光动力学分析来研究这些突变中的每一个如何影响应力诱导的 UBQLN2 凝聚体组装和凝聚体材料特性。与内源性 UBQLN2 不同,外源性引入的 UBQLN2 形成不同于应力颗粒的凝聚物。野生型和突变型 UBQLN2 缩合物通常是细胞质和液体状的。然而,与野生型 UBQLN2 相比,突变型 UBQLN2 形成更少的应激诱导的 UBQLN2 缩合物。外源表达的 P506T UBQLN2 在所有 UBQLN2 突变体中形成了最少数量的应激诱导凝聚物,并且这些凝聚物明显小于野生型 UBQLN2。UBQLN2 缩合物的光漂白 (FRAP) 分析后的荧光恢复显示 UBQLN2 突变体的固定分数更高,尤其是 P506T。P497S 和 P497H 突变对凝聚物特性的影响不同,表明 ALS 相关突变的影响是位置和氨基酸依赖性的。总的来说,我们的数据表明,疾病突变阻碍了应力诱导的 UBQLN2 缩合物的组装并改变了粘弹性,
更新日期:2021-06-15
中文翻译:

ALS相关突变损害UBQLN2应激诱导的细胞中生物分子凝聚物组装
ubiquilin-2 (UBQLN2) 是一种参与多种蛋白质质量控制过程的泛素结合穿梭蛋白,其突变可导致肌萎缩侧索硬化症 (ALS)。我们之前发现野生型 UBQLN2 在细胞应激下形成动态的、无膜的生物分子凝聚物,并在体外进行液-液相分离。然而,ALS 相关突变对细胞中 UBQLN2 凝聚物形成的影响仍然未知。在这里,我们用五个源自患者的 ALS 相关突变过表达 mCherry 融合的 UBQLN2,并采用活细胞成像和光动力学分析来研究这些突变中的每一个如何影响应力诱导的 UBQLN2 凝聚体组装和凝聚体材料特性。与内源性 UBQLN2 不同,外源性引入的 UBQLN2 形成不同于应力颗粒的凝聚物。野生型和突变型 UBQLN2 缩合物通常是细胞质和液体状的。然而,与野生型 UBQLN2 相比,突变型 UBQLN2 形成更少的应激诱导的 UBQLN2 缩合物。外源表达的 P506T UBQLN2 在所有 UBQLN2 突变体中形成了最少数量的应激诱导凝聚物,并且这些凝聚物明显小于野生型 UBQLN2。UBQLN2 缩合物的光漂白 (FRAP) 分析后的荧光恢复显示 UBQLN2 突变体的固定分数更高,尤其是 P506T。P497S 和 P497H 突变对凝聚物特性的影响不同,表明 ALS 相关突变的影响是位置和氨基酸依赖性的。总的来说,我们的数据表明,疾病突变阻碍了应力诱导的 UBQLN2 缩合物的组装并改变了粘弹性,











































 京公网安备 11010802027423号
京公网安备 11010802027423号