当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mutation of aspartic acid 199 in USP1 disrupts its deubiquitinating activity and impairs DNA repair
FEBS Letters ( IF 3.0 ) Pub Date : 2021-06-15 , DOI: 10.1002/1873-3468.14152 Seok Won Jang 1 , Jung Min Kim 1
FEBS Letters ( IF 3.0 ) Pub Date : 2021-06-15 , DOI: 10.1002/1873-3468.14152 Seok Won Jang 1 , Jung Min Kim 1
Affiliation
|
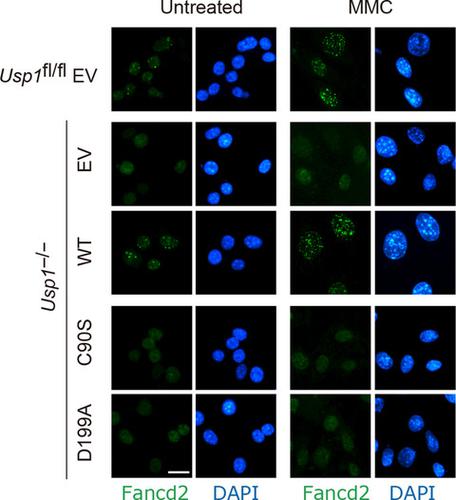
The deubiquitinating enzyme USP1 contains highly conserved motifs forming its catalytic center. Recently, the COSMIC mutation database identified a mutation in USP1 at Asp-199 in endometrial cancer. Here, we investigated the role of Asp-199 for USP1 function. The mutation of aspartic acid to alanine (D199A) resulted in failure of USP1 to undergo autocleavage and form a complex with ubiquitin, indicating D199A Usp1 is catalytically inactive. The D199A mutation did not affect the interaction with Uaf1. Moreover, D199A Usp1 had defects in deubiquitination of FANCD2 and PCNA and displayed reduced FANCD2 foci formation and DNA repair efficiency. Furthermore, mutation of Asp-199 to glutamic acid resulted in phenotypes similar to the D199A mutation. Collectively, our findings demonstrate the importance of Asp-199 for USP1 activity and suggest the implications of USP1 downregulation in cancer.
中文翻译:

USP1 中天冬氨酸 199 的突变破坏了其去泛素化活性并损害了 DNA 修复
去泛素化酶 USP1 包含形成其催化中心的高度保守的基序。最近,COSMIC 突变数据库在子宫内膜癌中发现了 USP1 中 Asp-199 的突变。在这里,我们研究了 Asp-199 对 USP1 功能的作用。天冬氨酸突变为丙氨酸 (D199A) 导致 USP1 无法进行自切割并与泛素形成复合物,表明 D199A Usp1 没有催化活性。D199A 突变不影响与 Uaf1 的相互作用。此外,D199A Usp1 在 FANCD2 和 PCNA 的去泛素化方面存在缺陷,并显示出 FANCD2 病灶形成和 DNA 修复效率降低。此外,Asp-199 突变为谷氨酸导致表型类似于 D199A 突变。总的来说,
更新日期:2021-08-09
中文翻译:

USP1 中天冬氨酸 199 的突变破坏了其去泛素化活性并损害了 DNA 修复
去泛素化酶 USP1 包含形成其催化中心的高度保守的基序。最近,COSMIC 突变数据库在子宫内膜癌中发现了 USP1 中 Asp-199 的突变。在这里,我们研究了 Asp-199 对 USP1 功能的作用。天冬氨酸突变为丙氨酸 (D199A) 导致 USP1 无法进行自切割并与泛素形成复合物,表明 D199A Usp1 没有催化活性。D199A 突变不影响与 Uaf1 的相互作用。此外,D199A Usp1 在 FANCD2 和 PCNA 的去泛素化方面存在缺陷,并显示出 FANCD2 病灶形成和 DNA 修复效率降低。此外,Asp-199 突变为谷氨酸导致表型类似于 D199A 突变。总的来说,











































 京公网安备 11010802027423号
京公网安备 11010802027423号