当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of benzene ring on the excited-state intramolecular proton transfer mechanisms of hydroxyquinoline derivatives
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2021-06-14 , DOI: 10.1002/poc.4257 Feng Zhang 1 , Jing Zhao 1 , Chaozheng Li 2
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2021-06-14 , DOI: 10.1002/poc.4257 Feng Zhang 1 , Jing Zhao 1 , Chaozheng Li 2
Affiliation
|
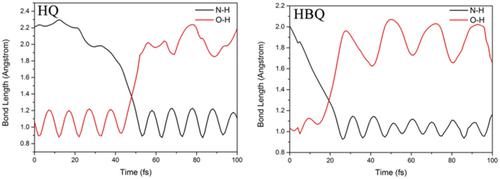
The proton transfer process of 8-hydroxyquinoline (HQ) can occur from the hydroxy oxygen to the pyridine nitrogen through a five-membered ring. For 10-hydroxybenzo[h]quinolone (HBQ), the proton transfer process occurs through a six-membered ring due to the insertion of a benzene ring between phenol and pyridine. In this work, the proton transfer processes of the five-membered (HQ) and six-membered (HBQ) ring hydroxyquinoline hydrogen bond systems were studied through theoretical methods. The geometric parameters and infrared (IR) vibrational spectra analysis show that the insertion of a benzene ring between phenol and pyridine affect the strength of the intramolecular hydrogen bond (O─H···N). Upon photoexcitation, the electron density is redistributed, which can provide driving force for the proton transfer processes. The potential barrier of the excited-state intramolecular proton transfer (ESIPT) process for HQ is 3.05 kcal/mol, whereas the ESIPT process of HBQ is barrierless. It is demonstrated that the proton transfer process of HBQ is easier than that of HQ in S1 state. The proton transfer process of HQ can only occur in S1 state, whereas that of HBQ can occur in the ground state (S0) and S1 state. In addition, the analysis of the nonadiabatic dynamics simulations reveals that the ESIPT process of HBQ (26 fs) is faster than that of HQ (53 fs). To sum up, the insertion of a benzene ring between phenol and pyridine affect the strength of the intramolecular hydrogen bond (O─H···N) and then affect the proton transfer processes to some extent.
中文翻译:

苯环对羟基喹啉衍生物激发态分子内质子转移机制的影响
8-羟基喹啉 (HQ) 的质子转移过程可以通过五元环从羟基氧到吡啶氮发生。对于 10-羟基苯并[ h]喹诺酮 (HBQ),由于苯环在苯酚和吡啶之间插入,质子转移过程通过六元环发生。在这项工作中,通过理论方法研究了五元(HQ)和六元(HBQ)环羟基喹啉氢键系统的质子转移过程。几何参数和红外(IR)振动光谱分析表明苯酚和吡啶之间插入苯环会影响分子内氢键(O─H…N)的强度。在光激发时,电子密度重新分布,这可以为质子转移过程提供驱动力。HQ 的激发态分子内质子转移 (ESIPT) 过程的势垒为 3.05 kcal/mol,而 HBQ 的 ESIPT 过程是无障碍的。1状态。HQ的质子转移过程只能发生在S 1态,而HBQ的质子转移过程可以发生在基态(S 0)和S 1态。此外,非绝热动力学模拟的分析表明,HBQ (26 fs) 的 ESIPT 过程比 HQ (53 fs) 快。综上所述,苯酚和吡啶之间插入苯环会影响分子内氢键(O─H…N)的强度,进而在一定程度上影响质子转移过程。
更新日期:2021-06-14
中文翻译:

苯环对羟基喹啉衍生物激发态分子内质子转移机制的影响
8-羟基喹啉 (HQ) 的质子转移过程可以通过五元环从羟基氧到吡啶氮发生。对于 10-羟基苯并[ h]喹诺酮 (HBQ),由于苯环在苯酚和吡啶之间插入,质子转移过程通过六元环发生。在这项工作中,通过理论方法研究了五元(HQ)和六元(HBQ)环羟基喹啉氢键系统的质子转移过程。几何参数和红外(IR)振动光谱分析表明苯酚和吡啶之间插入苯环会影响分子内氢键(O─H…N)的强度。在光激发时,电子密度重新分布,这可以为质子转移过程提供驱动力。HQ 的激发态分子内质子转移 (ESIPT) 过程的势垒为 3.05 kcal/mol,而 HBQ 的 ESIPT 过程是无障碍的。1状态。HQ的质子转移过程只能发生在S 1态,而HBQ的质子转移过程可以发生在基态(S 0)和S 1态。此外,非绝热动力学模拟的分析表明,HBQ (26 fs) 的 ESIPT 过程比 HQ (53 fs) 快。综上所述,苯酚和吡啶之间插入苯环会影响分子内氢键(O─H…N)的强度,进而在一定程度上影响质子转移过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号