当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of oxygen-bound reaction intermediates in selective electrochemical CO2 reduction
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2021-6-10 , DOI: 10.1039/d1ee00740h Xing Zhi 1, 2, 3, 4, 5 , Anthony Vasileff 1, 2, 3, 4, 5 , Yao Zheng 1, 2, 3, 4, 5 , Yan Jiao 1, 2, 3, 4, 5 , Shi-Zhang Qiao 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2021-6-10 , DOI: 10.1039/d1ee00740h Xing Zhi 1, 2, 3, 4, 5 , Anthony Vasileff 1, 2, 3, 4, 5 , Yao Zheng 1, 2, 3, 4, 5 , Yan Jiao 1, 2, 3, 4, 5 , Shi-Zhang Qiao 1, 2, 3, 4, 5
Affiliation
|
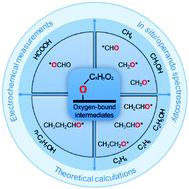
The electrochemical CO2 reduction reaction (CRR) is intrinsically complex given the multiple possible reaction pathways and end products. Consequently, selectivity is a persistent challenge for the design and operation of CRR electrocatalysts. A detailed understanding of key elementary steps and surface-bound species involved in the C1–C3 pathways is important for directing the reaction to a target product. However, there has been limited success in fully explaining the selectivity of CO2 reduction, such as the competing production of oxygen-free hydrocarbons and oxygen-containing alcohols. Recently, oxygen-bound intermediates have been identified as essential species to help explain the full reaction roadmap for CO2 reduction. This Review explores the important role of oxygen-bound intermediates in affecting CRR selectivity to the many reduction products, ranging from two electron products to higher reduced products. These oxygen-bound intermediates have a big influence on addressing mechanistic aspects of competing reaction pathways, based on extensive analysis of adsorption behaviour, reaction thermodynamics and reaction kinetics. Considering available theoretical calculations, electrochemical measurements and operando spectroscopy observations, we highlight the preferred reaction pathways to certain products when regulated by oxygen-bound species. The geometries of these oxygen-bound intermediates and their binding on a catalyst surface dictate the breakage or preservation of C–O bonds, which has a significant effect on directing selectivity toward a final product. Based on this mechanistic evaluation, we summarize practical techniques for probing the evolution of intermediates and propose possible strategies for promoting the selectivity of electrocatalysts.
中文翻译:

氧结合反应中间体在选择性电化学 CO2 还原中的作用
考虑到多种可能的反应途径和最终产物,电化学 CO 2还原反应 (CRR) 本质上是复杂的。因此,选择性是 CRR 电催化剂设计和操作的持续挑战。详细了解 C 1 -C 3途径中涉及的关键基本步骤和表面结合物质对于将反应导向目标产物非常重要。然而,在充分解释 CO 2还原的选择性方面取得的成功有限,例如无氧烃和含氧醇的竞争生产。最近,氧结合中间体已被确定为必要物质,以帮助解释 CO 2的完整反应路线图减少。本综述探讨了氧结合中间体在影响 CRR 对许多还原产物(从两个电子产物到更高还原产物)的选择性方面的重要作用。基于对吸附行为、反应热力学和反应动力学的广泛分析,这些与氧结合的中间体对解决竞争反应途径的机械方面有很大影响。考虑可用的理论计算、电化学测量和操作在光谱观察中,我们强调了某些产物在受氧结合物质调节时的首选反应途径。这些氧结合中间体的几何形状及其在催化剂表面上的结合决定了 C-O 键的断裂或保存,这对将选择性导向最终产品具有重要影响。基于这种机理评估,我们总结了探索中间体演化的实用技术,并提出了提高电催化剂选择性的可能策略。
更新日期:2021-06-15
中文翻译:

氧结合反应中间体在选择性电化学 CO2 还原中的作用
考虑到多种可能的反应途径和最终产物,电化学 CO 2还原反应 (CRR) 本质上是复杂的。因此,选择性是 CRR 电催化剂设计和操作的持续挑战。详细了解 C 1 -C 3途径中涉及的关键基本步骤和表面结合物质对于将反应导向目标产物非常重要。然而,在充分解释 CO 2还原的选择性方面取得的成功有限,例如无氧烃和含氧醇的竞争生产。最近,氧结合中间体已被确定为必要物质,以帮助解释 CO 2的完整反应路线图减少。本综述探讨了氧结合中间体在影响 CRR 对许多还原产物(从两个电子产物到更高还原产物)的选择性方面的重要作用。基于对吸附行为、反应热力学和反应动力学的广泛分析,这些与氧结合的中间体对解决竞争反应途径的机械方面有很大影响。考虑可用的理论计算、电化学测量和操作在光谱观察中,我们强调了某些产物在受氧结合物质调节时的首选反应途径。这些氧结合中间体的几何形状及其在催化剂表面上的结合决定了 C-O 键的断裂或保存,这对将选择性导向最终产品具有重要影响。基于这种机理评估,我们总结了探索中间体演化的实用技术,并提出了提高电催化剂选择性的可能策略。









































 京公网安备 11010802027423号
京公网安备 11010802027423号