当前位置:
X-MOL 学术
›
Can. J. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhancement of kinetic rates of CO2 in aqueous solutions of piperazine and methyldiethanolamine with addition of sulfolane
The Canadian Journal of Chemical Engineering ( IF 1.6 ) Pub Date : 2021-06-11 , DOI: 10.1002/cjce.24222 Abdelmouiz Ahmed 1 , Amr Henni 2 , Abdenacer Guibadj 1 , Ahmed Hadjadj 3
The Canadian Journal of Chemical Engineering ( IF 1.6 ) Pub Date : 2021-06-11 , DOI: 10.1002/cjce.24222 Abdelmouiz Ahmed 1 , Amr Henni 2 , Abdenacer Guibadj 1 , Ahmed Hadjadj 3
Affiliation
|
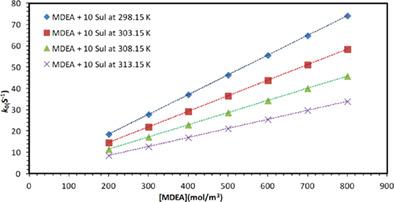
Measurements of kinetics rates of CO2 in aqueous solutions of methyldiethanolamine (MDEA), piperazine (PZ), and mixtures of (MDEA + PZ), (PZ + sulfolane) and (MDEA + sulfolane) were carried out using the stopped flow technique, and reported in terms of pseudo-first-order rate constants (k0). When possible, the second-order reaction rate constants (k2) were regressed from the data. Experiments were performed over new concentration ranges of (10–60), (200–800), (200–800, 10–40), (10–40, 10–200), and (200–800, 10–200) mol/m3 for the above-mentioned five systems, respectively, and at temperatures varying from (298.15–313.15 K). When sulfolane was added to the amine solution, pseudo-first-order rate constants in the mixed solvents were higher than in aqueous MDEA and PZ solutions at all temperatures. The kinetic rates were highest at 298.15 K and decreased at higher temperatures for aqueous (MDEA + sulfolane) solutions but increased with temperature for aqueous (PZ + sulfolane) systems. Reaction orders for both PZ and MDEA were practically one at all sulfolane concentrations and temperatures. The base catalysis mechanism was used to regress very well data for aqueous MDEA and (MDEA + sulfolane + water) and the termolecular mechanism was used for (PZ + sulfolane + water) system. Both the zwitterion and termolecular models were able to fit the experimental data for the aqueous PZ system well. Finally, the termolecular and a hybrid model based on the combination of the Zwitterion and base catalysis mechanisms were able to successfully correlate the experimental data for the mixed aqueous (MDEA + PZ) systems.
中文翻译:

添加环丁砜可提高哌嗪和甲基二乙醇胺水溶液中 CO2 的动力学速率
使用停流技术测量甲基二乙醇胺 (MDEA)、哌嗪 (PZ) 和 (MDEA + PZ)、(PZ + 环丁砜) 和 (MDEA + 环丁砜) 的混合物中 CO 2的动力学速率,并以伪一阶速率常数 ( k 0 ) 表示。在可能的情况下,二级反应速率常数 ( k 2 ) 从数据中回归。在 (10–60)、(200–800)、(200–800, 10–40)、(10–40, 10–200) 和 (200–800, 10–200) 的新浓度范围内进行实验摩尔/米3对于上述五个系统,分别在 (298.15–313.15 K) 的温度下。当将环丁砜添加到胺溶液中时,混合溶剂中的准一级速率常数在所有温度下都高于 MDEA 和 PZ 水溶液。动力学速率在 298.15 K 时最高,水溶液(MDEA + 环丁砜)溶液的动力学速率在较高温度下降低,但水溶液(PZ + 环丁砜)系统的动力学速率随温度升高。在所有环丁砜浓度和温度下,PZ 和 MDEA 的反应顺序实际上都是一个。碱催化机制用于对含水 MDEA 和(MDEA + 环丁砜 + 水)的数据进行很好的回归,而双分子机制用于(PZ + 环丁砜 + 水)系统。两性离子和双分子模型都能够很好地拟合水性 PZ 系统的实验数据。最后,基于两性离子和碱催化机制相结合的双分子和混合模型能够成功地关联混合水(MDEA + PZ)系统的实验数据。
更新日期:2021-06-11
中文翻译:

添加环丁砜可提高哌嗪和甲基二乙醇胺水溶液中 CO2 的动力学速率
使用停流技术测量甲基二乙醇胺 (MDEA)、哌嗪 (PZ) 和 (MDEA + PZ)、(PZ + 环丁砜) 和 (MDEA + 环丁砜) 的混合物中 CO 2的动力学速率,并以伪一阶速率常数 ( k 0 ) 表示。在可能的情况下,二级反应速率常数 ( k 2 ) 从数据中回归。在 (10–60)、(200–800)、(200–800, 10–40)、(10–40, 10–200) 和 (200–800, 10–200) 的新浓度范围内进行实验摩尔/米3对于上述五个系统,分别在 (298.15–313.15 K) 的温度下。当将环丁砜添加到胺溶液中时,混合溶剂中的准一级速率常数在所有温度下都高于 MDEA 和 PZ 水溶液。动力学速率在 298.15 K 时最高,水溶液(MDEA + 环丁砜)溶液的动力学速率在较高温度下降低,但水溶液(PZ + 环丁砜)系统的动力学速率随温度升高。在所有环丁砜浓度和温度下,PZ 和 MDEA 的反应顺序实际上都是一个。碱催化机制用于对含水 MDEA 和(MDEA + 环丁砜 + 水)的数据进行很好的回归,而双分子机制用于(PZ + 环丁砜 + 水)系统。两性离子和双分子模型都能够很好地拟合水性 PZ 系统的实验数据。最后,基于两性离子和碱催化机制相结合的双分子和混合模型能够成功地关联混合水(MDEA + PZ)系统的实验数据。









































 京公网安备 11010802027423号
京公网安备 11010802027423号