当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel 4-Hydroxybenzyl Adducts in Human Hemoglobin: Structures and Mechanisms of Formation
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2021-06-10 , DOI: 10.1021/acs.chemrestox.1c00111 Andrew T Rajczewski 1 , Lorena Ndreu 2 , Suresh S Pujari 3 , Timothy J Griffin 1 , Margareta Å Törnqvist 2 , Isabella Karlsson 2 , Natalia Y Tretyakova 3
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2021-06-10 , DOI: 10.1021/acs.chemrestox.1c00111 Andrew T Rajczewski 1 , Lorena Ndreu 2 , Suresh S Pujari 3 , Timothy J Griffin 1 , Margareta Å Törnqvist 2 , Isabella Karlsson 2 , Natalia Y Tretyakova 3
Affiliation
|
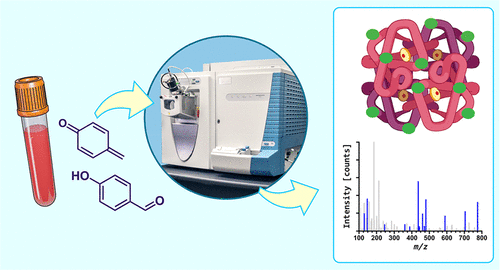
Humans are exposed to large numbers of electrophiles from their diet, the environment, and endogenous physiological processes. Adducts formed at the N-terminal valine of hemoglobin are often used as biomarkers of human exposure to electrophilic compounds. We previously reported the formation of hemoglobin N-terminal valine adducts (added mass, 106.042 Da) in the blood of human smokers and nonsmokers and identified their structure as 4-hydroxybenzyl-Val. In the present work, mass spectrometry-based proteomics was utilized to identify additional sites for 4-hydroxybenzyl adduct formation at internal nucleophilic amino acid side chains within hemoglobin. Hemoglobin isolated from human blood was treated with para-quinone methide (para-QM) followed by global nanoLC-MS/MS and targeted nanoLC-MS/MS to identify amino acid residues containing the 4-hydroxybenzyl modification. Our experiments revealed the formation of 4-hydroxybenzyl adducts at the αHis20, αTyr24, αTyr42, αHis45, βSer72, βThr84, βThr87, βSer89, βHis92, βCys93, βCys112, βThr123, and βHis143 residues (in addition to N-terminal valine) through characteristic MS/MS spectra. These amino acid side chains had variable reactivity toward para-QM with αHis45, αTyr42, βCys93, βHis92, and βSer72 forming the largest numbers of adducts upon exposure to para-QM. Two additional mechanisms for formation of 4-hydroxybenzyl adducts in humans were investigated: exposure to 4-hydroxybenzaldehyde (4-HBA) followed by reduction and UV-mediated reactions of hemoglobin with tyrosine. Exposure of hemoglobin to a 5-fold molar excess of 4-HBA followed by reduction with sodium cyanoborohydride produced 4-hydroxybenzyl adducts at several amino acid side chains of which αHis20, αTyr24, αTyr42, αHis45, βSer44, βThr84, and βHis92 were verified in targeted mass spectrometry experiments. Similarly, exposure of human blood to ultraviolet radiation produced 4-hydroxybenzyl adducts at αHis20, αTyr24, αTyr42, αHis45, βSer44, βThr84, and βSer89. Overall, our results reveal that 4-hydroxybenzyl adducts form at multiple nucleophilic sites of hemoglobin and that para-QM is the most likely source of these adducts in humans.
中文翻译:

人类血红蛋白中的新型 4-羟基苄基加合物:结构和形成机制
人类从饮食、环境和内源性生理过程中接触到大量亲电子试剂。在血红蛋白的 N 末端缬氨酸形成的加合物通常用作人类暴露于亲电子化合物的生物标志物。我们之前报道了人类吸烟者和非吸烟者血液中血红蛋白 N-末端缬氨酸加合物(添加质量,106.042 Da)的形成,并将其结构鉴定为 4-羟基苄基-Val。在目前的工作中,基于质谱的蛋白质组学被用于识别血红蛋白内部亲核氨基酸侧链上 4-羟基苄基加合物形成的其他位点。从人体血液中分离出的血红蛋白用对苯醌甲基化物(para-QM),然后是全局 nanoLC-MS/MS 和靶向 nanoLC-MS/MS,以鉴定含有 4-羟基苄基修饰的氨基酸残基。我们的实验揭示了在 αHis20、αTyr24、αTyr42、αHis45、βSer72、βThr84、βThr87、βSer89、βHis92、βCys93、βCys112、βThr123 和 βHis143 残基(除了 N -末端缬氨酸)通过特征MS/MS 光谱。这些氨基酸侧链对para -QM 具有不同的反应性,其中 αHis45、αTyr42、βCys93、βHis92 和 βSer72 在暴露于para时形成最大数量的加合物-质量管理。研究了在人体中形成 4-羟基苄基加合物的另外两种机制:暴露于 4-羟基苯甲醛 (4-HBA),然后还原和紫外线介导的血红蛋白与酪氨酸的反应。血红蛋白暴露于 5 倍摩尔过量的 4-HBA,然后用氰基硼氢化钠还原在几个氨基酸侧链上产生 4-羟基苄基加合物,其中 αHis20、αTyr24、αTyr42、αHis45、βSer44、βThr84 和 βHis92 在靶向质谱实验。同样,人体血液暴露于紫外线辐射会在 αHis20、αTyr24、αTyr42、αHis45、βSer44、βThr84 和 βSer89 处产生 4-羟基苄基加合物。总的来说,我们的结果表明 4-羟基苄基加合物在血红蛋白的多个亲核位点形成,并且对位-QM 是人体中这些加合物最有可能的来源。
更新日期:2021-07-19
中文翻译:

人类血红蛋白中的新型 4-羟基苄基加合物:结构和形成机制
人类从饮食、环境和内源性生理过程中接触到大量亲电子试剂。在血红蛋白的 N 末端缬氨酸形成的加合物通常用作人类暴露于亲电子化合物的生物标志物。我们之前报道了人类吸烟者和非吸烟者血液中血红蛋白 N-末端缬氨酸加合物(添加质量,106.042 Da)的形成,并将其结构鉴定为 4-羟基苄基-Val。在目前的工作中,基于质谱的蛋白质组学被用于识别血红蛋白内部亲核氨基酸侧链上 4-羟基苄基加合物形成的其他位点。从人体血液中分离出的血红蛋白用对苯醌甲基化物(para-QM),然后是全局 nanoLC-MS/MS 和靶向 nanoLC-MS/MS,以鉴定含有 4-羟基苄基修饰的氨基酸残基。我们的实验揭示了在 αHis20、αTyr24、αTyr42、αHis45、βSer72、βThr84、βThr87、βSer89、βHis92、βCys93、βCys112、βThr123 和 βHis143 残基(除了 N -末端缬氨酸)通过特征MS/MS 光谱。这些氨基酸侧链对para -QM 具有不同的反应性,其中 αHis45、αTyr42、βCys93、βHis92 和 βSer72 在暴露于para时形成最大数量的加合物-质量管理。研究了在人体中形成 4-羟基苄基加合物的另外两种机制:暴露于 4-羟基苯甲醛 (4-HBA),然后还原和紫外线介导的血红蛋白与酪氨酸的反应。血红蛋白暴露于 5 倍摩尔过量的 4-HBA,然后用氰基硼氢化钠还原在几个氨基酸侧链上产生 4-羟基苄基加合物,其中 αHis20、αTyr24、αTyr42、αHis45、βSer44、βThr84 和 βHis92 在靶向质谱实验。同样,人体血液暴露于紫外线辐射会在 αHis20、αTyr24、αTyr42、αHis45、βSer44、βThr84 和 βSer89 处产生 4-羟基苄基加合物。总的来说,我们的结果表明 4-羟基苄基加合物在血红蛋白的多个亲核位点形成,并且对位-QM 是人体中这些加合物最有可能的来源。











































 京公网安备 11010802027423号
京公网安备 11010802027423号