当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
MagC is a NplC/P60-like member of the α-2-macroglobulin Mag complex of Pseudomonas aeruginosa that interacts with peptidoglycan
FEBS Letters ( IF 3.0 ) Pub Date : 2021-06-11 , DOI: 10.1002/1873-3468.14148 Samira Zouhir 1 , Carlos Contreras-Martel 2 , Daniel Maragno Trindade 1 , Ina Attrée 3 , Andréa Dessen 1, 2 , Pauline Macheboeuf 2
FEBS Letters ( IF 3.0 ) Pub Date : 2021-06-11 , DOI: 10.1002/1873-3468.14148 Samira Zouhir 1 , Carlos Contreras-Martel 2 , Daniel Maragno Trindade 1 , Ina Attrée 3 , Andréa Dessen 1, 2 , Pauline Macheboeuf 2
Affiliation
|
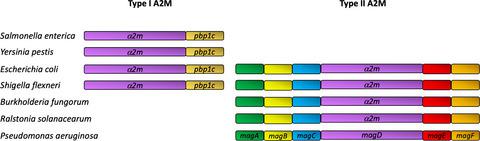
Bacterial α-2 macroglobulins (A2Ms) structurally resemble the large spectrum protease inhibitors of the eukaryotic immune system. In Pseudomonas aeruginosa, MagD acts as an A2M and is expressed within a six-gene operon encoding the MagA-F proteins. In this work, we employ isothermal calorimetry (ITC), analytical ultracentrifugation (AUC), and X-ray crystallography to investigate the function of MagC and show that MagC associates with the macroglobulin complex and with the peptidoglycan (PG). However, the catalytic residues of MagC display an inactive conformation that could suggest that it binds to PG but does not degrade it. We hypothesize that MagC could serve as an anchor between the MagD macroglobulin and the PG and could provide stabilization and/or regulation for the entire complex.
中文翻译:

MagC 是铜绿假单胞菌的 α-2-巨球蛋白 Mag 复合物的 NplC/P60 样成员,与肽聚糖相互作用
细菌 α-2 巨球蛋白 (A2Ms) 在结构上类似于真核免疫系统的广谱蛋白酶抑制剂。在铜绿假单胞菌中,MagD 充当 A2M 并在编码 MagA-F 蛋白的六基因操纵子内表达。在这项工作中,我们采用等温量热法 (ITC)、分析超速离心法 (AUC) 和 X 射线晶体学来研究 MagC 的功能,并表明 MagC 与巨球蛋白复合物和肽聚糖 (PG) 相关联。然而,MagC 的催化残基显示出一种无活性的构象,这可能表明它与 PG 结合但不会降解它。我们假设 MagC 可以作为 MagD 巨球蛋白和 PG 之间的锚点,并且可以为整个复合物提供稳定和/或调节。
更新日期:2021-08-09
中文翻译:

MagC 是铜绿假单胞菌的 α-2-巨球蛋白 Mag 复合物的 NplC/P60 样成员,与肽聚糖相互作用
细菌 α-2 巨球蛋白 (A2Ms) 在结构上类似于真核免疫系统的广谱蛋白酶抑制剂。在铜绿假单胞菌中,MagD 充当 A2M 并在编码 MagA-F 蛋白的六基因操纵子内表达。在这项工作中,我们采用等温量热法 (ITC)、分析超速离心法 (AUC) 和 X 射线晶体学来研究 MagC 的功能,并表明 MagC 与巨球蛋白复合物和肽聚糖 (PG) 相关联。然而,MagC 的催化残基显示出一种无活性的构象,这可能表明它与 PG 结合但不会降解它。我们假设 MagC 可以作为 MagD 巨球蛋白和 PG 之间的锚点,并且可以为整个复合物提供稳定和/或调节。











































 京公网安备 11010802027423号
京公网安备 11010802027423号